Porphyrin-based compounds for tumor imaging and photodynamic therapy
A technology of compound and porphyrin, which is applied in the field of porphyrin-based compounds for tumor imaging and photodynamic therapy, can solve the problems of deep malignant lesions, impractical PDT, and inaccessibility of singlet oxygen product light, and achieve high The effect of tumor uptake
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
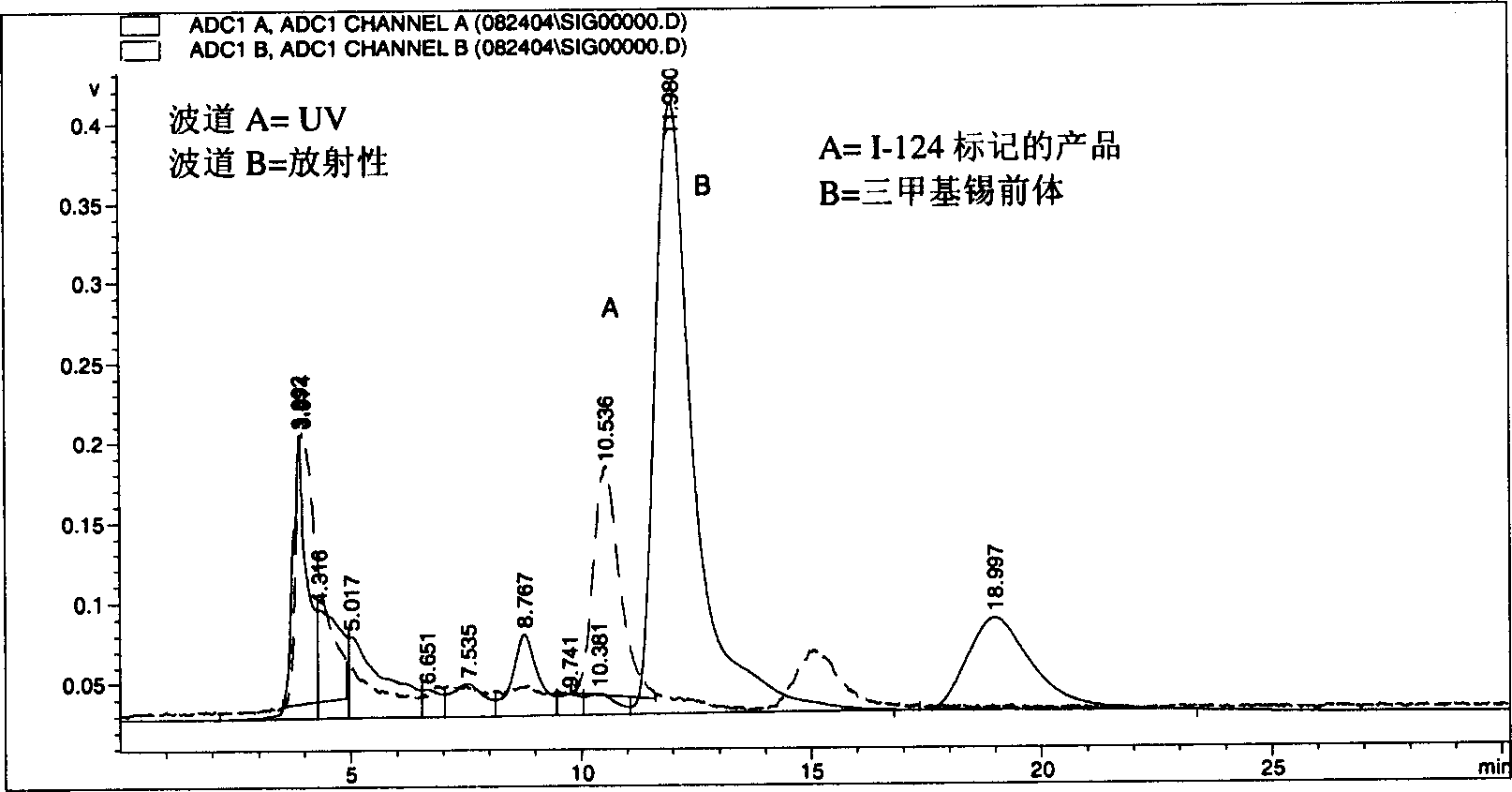
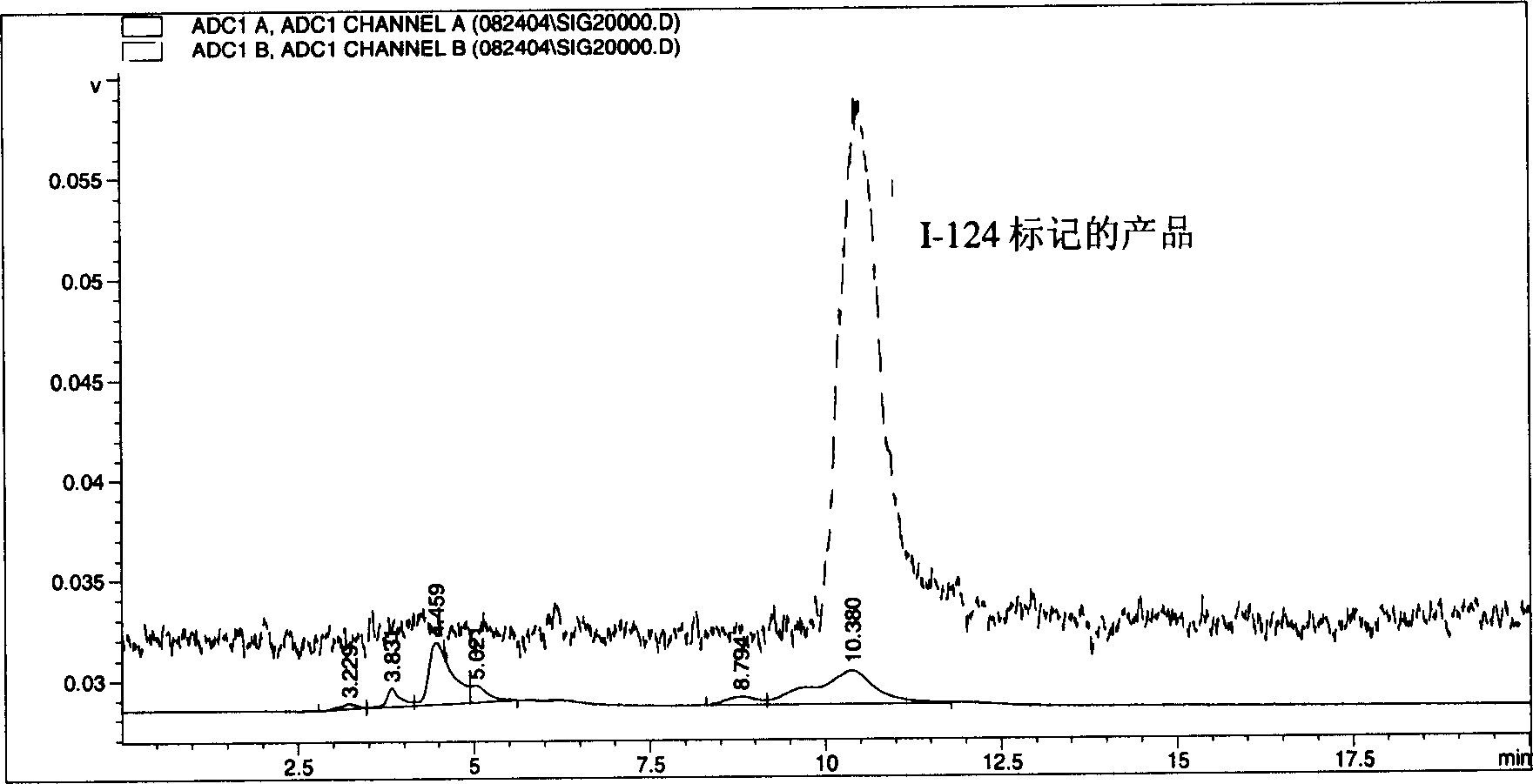
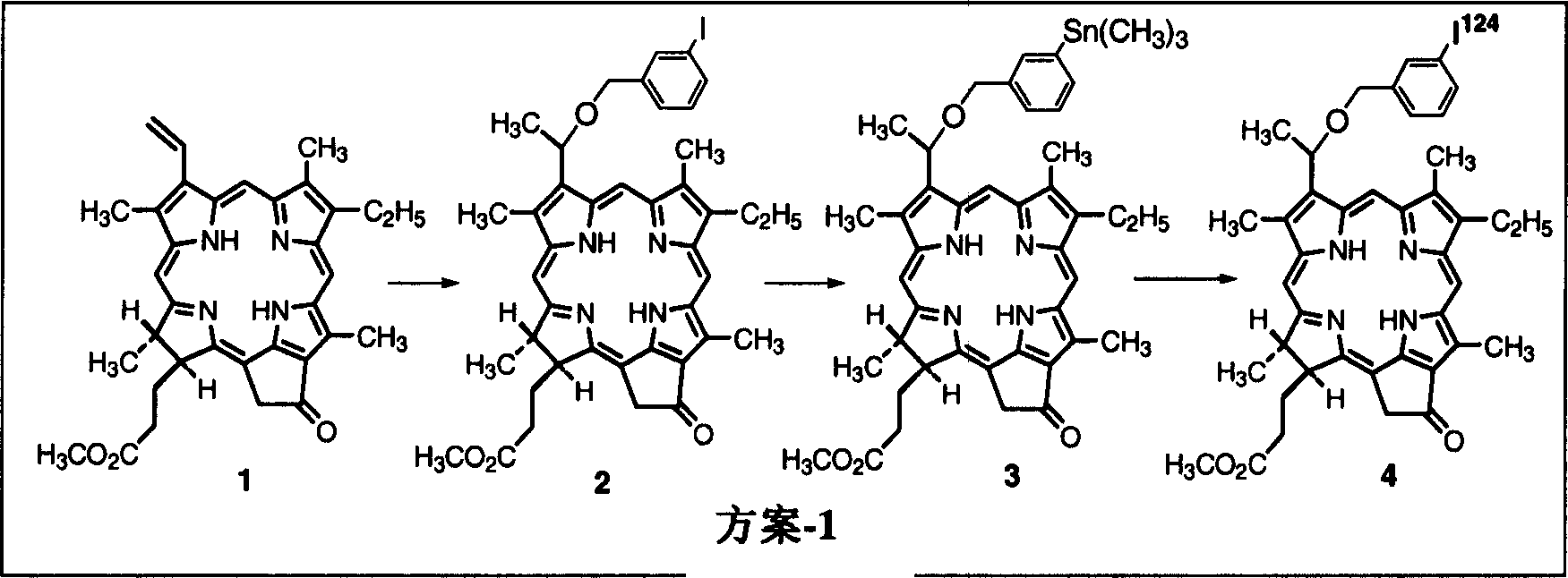
[0034] Based on the research on a series of alkyl ether analogues of pyropheophorbide-a, the inventors developed a photosensitizer that absorbs relatively long wavelengths, 3-(1-hexyl oxy)ethyl-derivatives (HPPH). The compound is tumor-avid and is currently in Phase I / II human clinical trials at the Roswell Park Cancer Institute. The inventors have studied this compound and single-or bis-diaminoethanethiol (N 2 S 2 Ligand) in conjunction with use as a "vehicle". Results obtained from in vivo biodistribution assays indicated that tumor / non-tumor uptake rates of drugs depended on time and tumor size. The clearance of HPPH-based compounds from tumors was found to be slower in time than from most non-tumor tissues. However, found 99m The short 6-hour half-life of Tc does not match the 24-hour imaging time, suggesting that the use of longer-lived isotopes could provide useful scanning agents. Another approach to the development of improved tumor imaging agents could be to rep...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com