Carbonaceous lithium ion battery negative electrode material with nuclear shell structure and producing method thereof
A technology for lithium ion batteries and negative electrode materials, which is applied to battery electrodes, structural parts, circuits, etc., can solve the problems of poor cycle performance, high first irreversible amount, and reduced first irreversible amount, etc., and achieve long cycle life and low first irreversible capacity. , the effect of high capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] a) Ordinary H prepared by chemical method 2 SO 4 -H 2 o 2 The system expandable graphite is pulverized to a particle size of 5-50 μm.
[0032] b) Heating the obtained graphite intercalation compound at 250° C. for 48 hours to slowly deintercalate.
[0033] c) Prepare a 3 wt% ethanol solution of phenolic resin, immerse the deintercalated graphite in the solution, stir for 6 hours, let stand for 4 hours, filter, and dry the filtered graphite powder at 300°C.
[0034] d) heating the graphite powder obtained in the previous step to 1000° C. at a rate of 300° C. / h under nitrogen protection, keeping the temperature for 4 hours, and cooling with the furnace to obtain graphite powder coated with amorphous carbon on the surface.
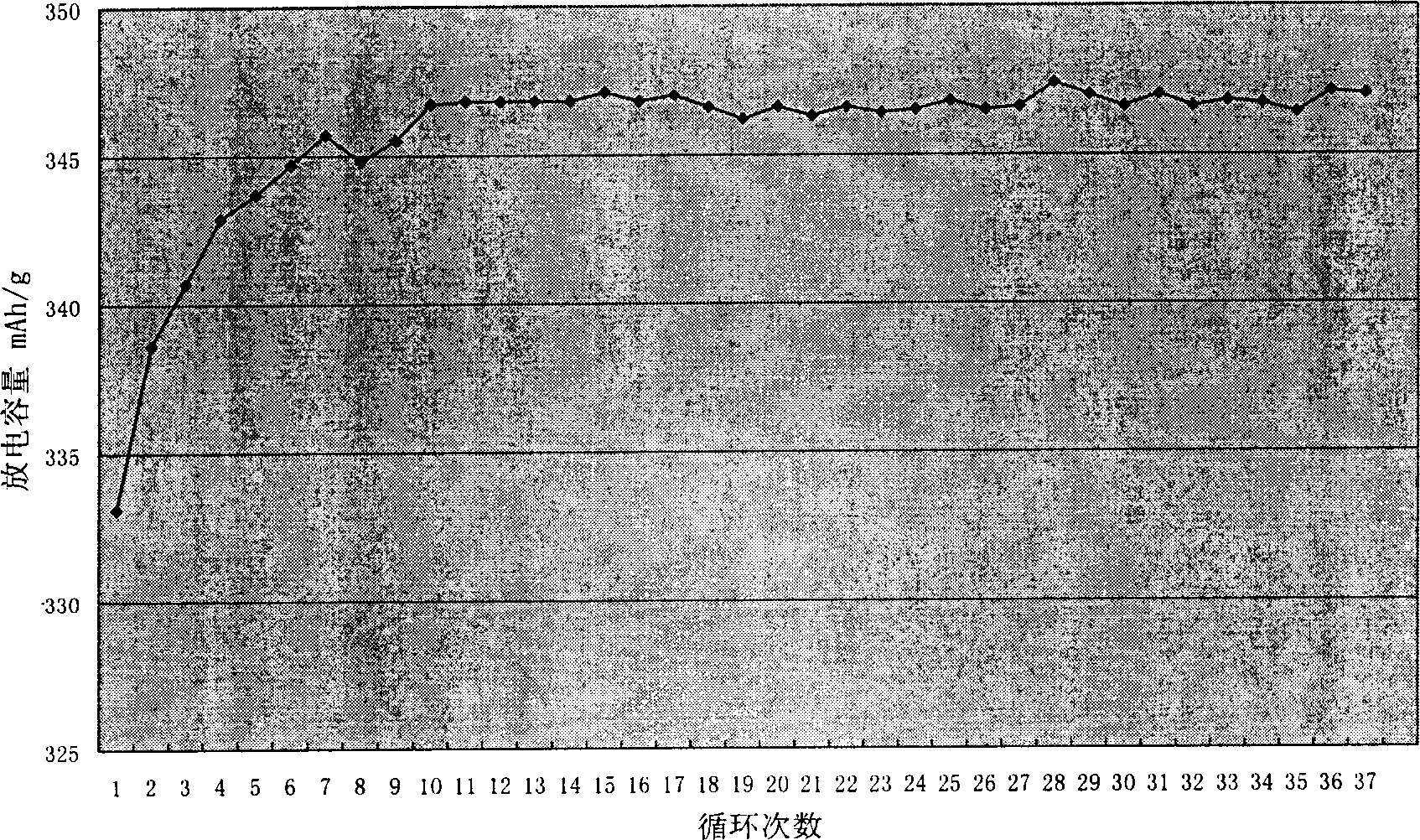
[0035] The first cycle efficiency of the graphite powder coated with amorphous carbon on the surface obtained by this method is greater than 90%, and the test results of 38 cycles of discharge capacity change are as follows:
Embodiment 2
[0037] a) FeCl prepared by electrochemical method 3 The intercalated expandable graphite is pulverized to a particle size of 5-50 μm.
[0038] b) Heating the obtained graphite intercalation compound at 350° C. for 72 h to slowly deintercalate.
[0039] c) Prepare 20 wt% sucrose aqueous solution, immerse the deintercalated graphite into the solution, stir for 0.5 hours, let stand for 6 hours, filter, and dry the filtered graphite powder at 100°C.
[0040] d) The graphite powder obtained in the previous step is heated up to 700° C. at a rate of 500° C. / h under the protection of nitrogen, kept at a temperature of 0.5 hours, and cooled with the furnace to obtain graphite powder coated with amorphous carbon on the surface.
Embodiment 3
[0042] a) flake graphite powder with a particle size of 5-50 μm is prepared by chemical method 2 SO 4 Intercalated graphite intercalation compound.
[0043] b) Heat the obtained graphite intercalation compound at 205° C. for 12 hours, and slowly deintercalate it.
[0044] c) Prepare 5% mass fraction of phenolic resin ethanol solution, immerse the deintercalated graphite in the solution, stir for 2 hours, let stand for 6 hours, filter, and dry the filtered graphite powder at 200°C.
[0045] d) heating the graphite powder obtained in the previous step to 1000° C. at a rate of 250° C. / h under nitrogen protection, keeping the temperature for 3 hours, and cooling with the furnace to obtain graphite powder coated with amorphous carbon on the surface.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com