Liquid phase chip for parallel detection of autoantibodies, preparation method and application thereof
An autoantibody, parallel detection technology, applied in the field of immune technology and clinical detection, can solve problems such as inconvenience, and achieve the effect of saving detection costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1: Coupling of self-antigens to known numbered microspheres
[0024] 1. Select No. 28, 38, 48, 58, 68, and 78 carboxyl microspheres (Luminex Company) respectively, and oscillate the microsphere suspension with a vortex shaker for 20 seconds to mix the microspheres evenly.
[0025] 2. Take about 2×10 carboxyl microspheres of each number above. 3 Transfer each to a centrifuge tube and centrifuge at ≥8000×g for 2 min to precipitate carboxylated microspheres.
[0026] 3. Remove the supernatant and add 100μl dH 2 O, use a vortex shaker to resuspend the microspheres for 20 seconds, centrifuge at ≥8000×g for 2 minutes, and precipitate the carboxylated microspheres.
[0027]4. Remove the supernatant, add 80 μl, 100 mM, pH=6.2 sodium dihydrogen phosphate salt solution, and resuspend the washed carboxy microspheres with a vortex shaker for 20 seconds.
[0028] 5. Add 10μl, 50mg / ml Sulfo-NHS (with dH 2 O dilution), oscillate gently with a vortex shaker.
[0029] 6. Ad...
Embodiment 2
[0040] Example 2: Application of liquid phase chip for parallel detection of autoimmune diseases described in the present invention in clinical detection
[0041] (1) The technical process of using the autoantibody parallel detection liquid phase chip of the present invention to detect autoantibodies:
[0042] 1. Take out 500 microspheres conjugated to each autoantigen prepared above, mix them in equal proportions, and distribute them in 96-well plates. Each well contains 500 microspheres conjugated to various autoantibodies. Add 50 μl of serum to be tested and incubate at 37°C for 2 hours.
[0043] 2. Centrifuge at ≥8000×g for 2 minutes, remove the supernatant, add 300 μl of 1% PBS-BSA, vortex for 30 seconds, and seal in a 37°C incubator for 1 hour.
[0044] 3. Centrifuge at ≥8000×g for 2 minutes. Remove the supernatant, add 300 μl PBS-TBN, and vortex for 30 seconds. Centrifuge at ≥8000×g for 2 minutes.
[0045] 4. Repeat step 3 twice.
[0046] 5. Add 100 μl of the bioti...
Embodiment 3
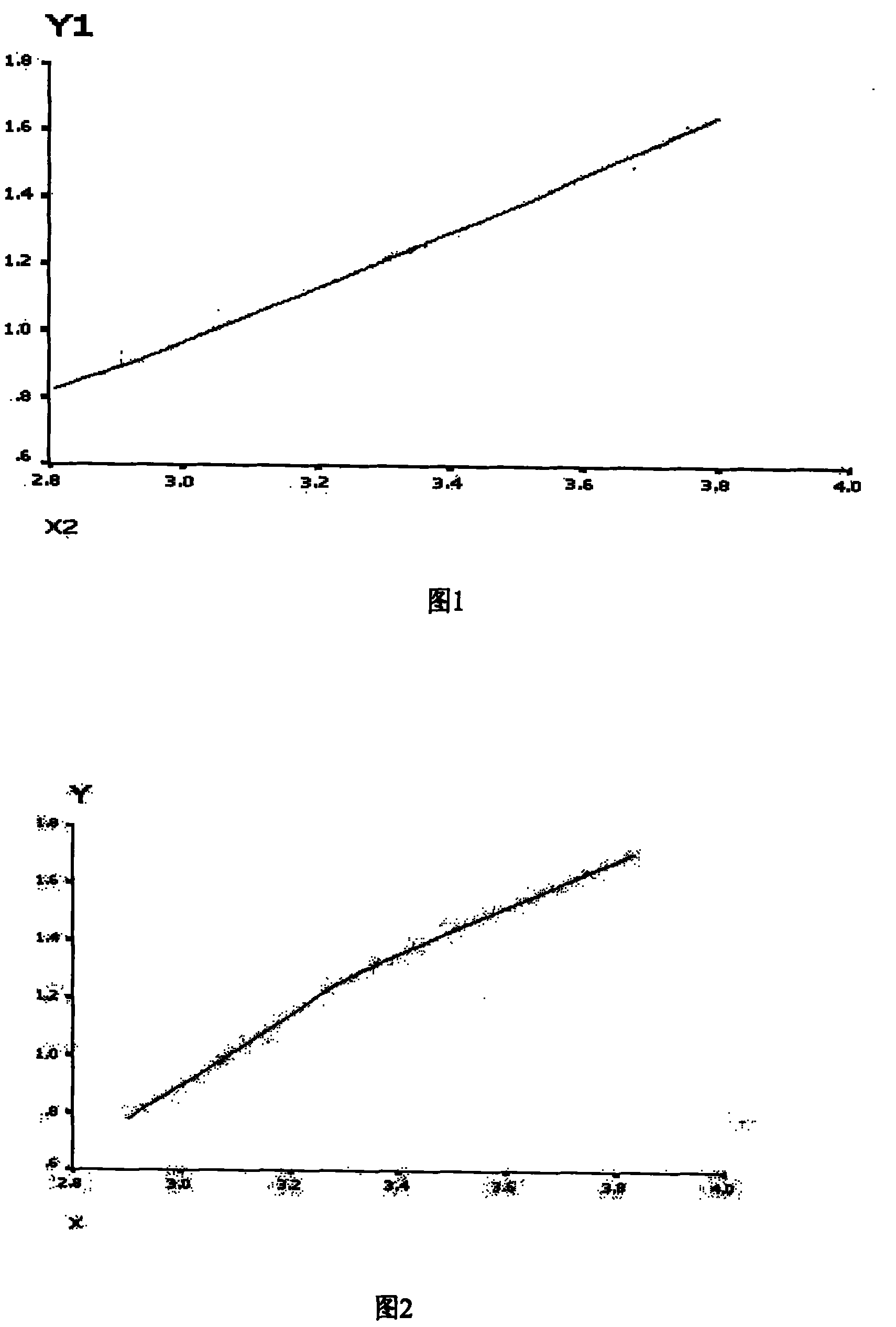
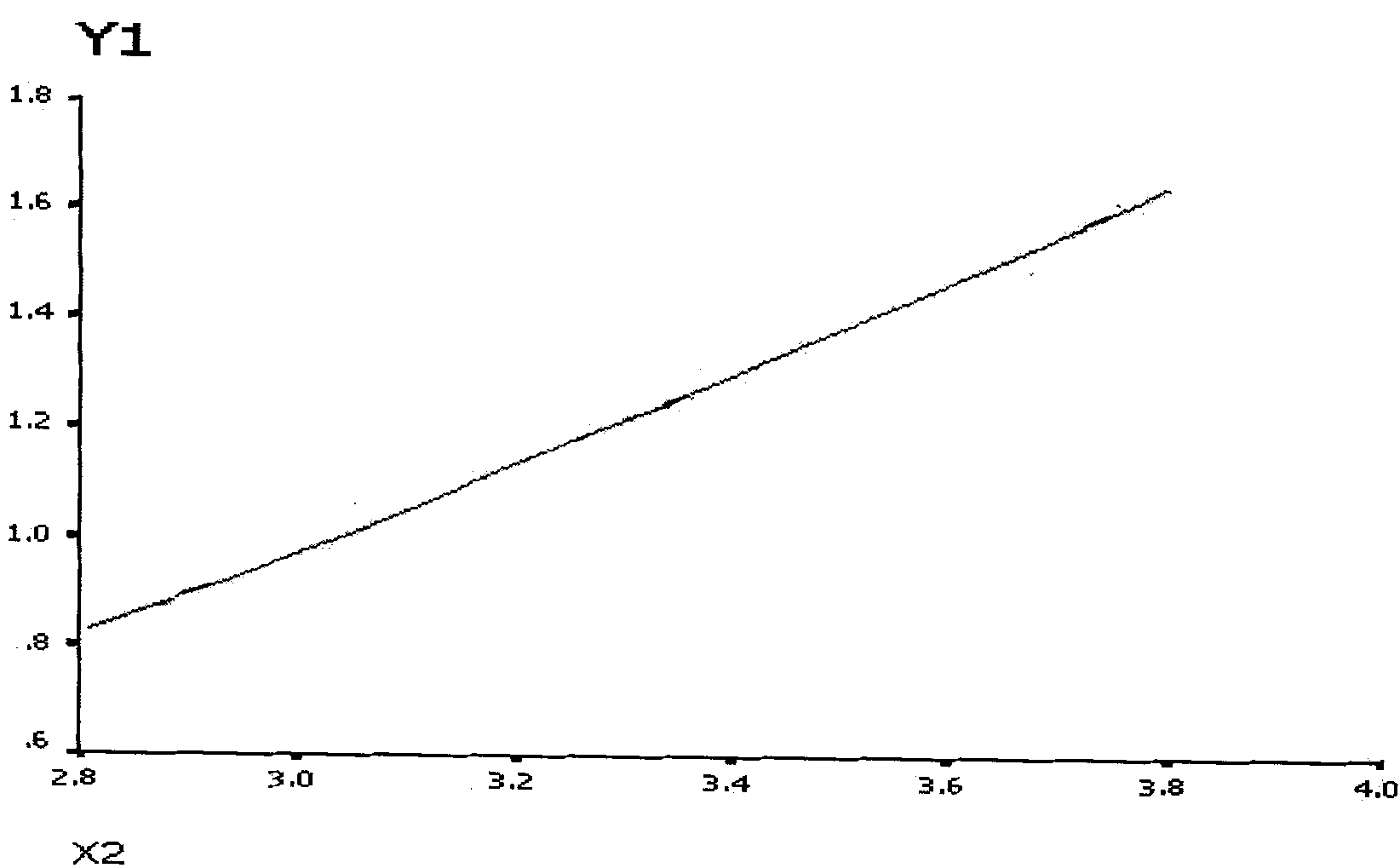
[0055] Example 3: Example 1 of standard curve drawing of autoantibodies
[0056] According to the method described in Examples 1 and 2, SSA / Ro-52 (RO52) was coated onto No. 38 microspheres. After coating, 1000 microspheres were added to PE-labeled mouse anti-human IgG to react for 30 minutes, and detected by Luminex100 , the fluorescence value reached 23,000, while the microspheres not coated with SSA / Ro-52 were reacted with PE-labeled mouse anti-human IgG for 30 minutes. After Luminex100 detection, the fluorescence value was only 100, indicating that our coated microspheres can meet the needs of chip development. requirements.
[0057] Take the standard SSA / Ro-52 antibody, dilute it according to the gradient of 0EU / ml, 6.25EU / ml, 12.5EU / ml, 25EU / ml and 50EU / ml, react according to the method described, and detect by Luminex100, the average fluorescence intensity is respectively for 261.5, 908, 1475, 4422 and 6654.5. For the standard curve obtained, see figure 1 . One case ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| fluorescence | aaaaa | aaaaa |
| fluorescence | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com