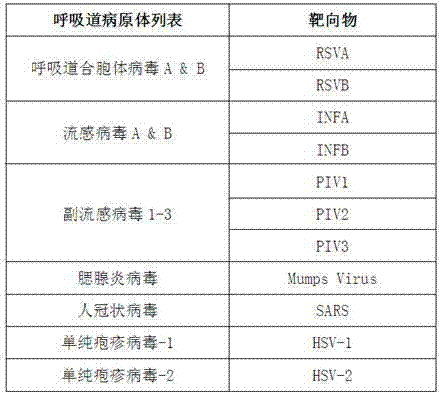
Nucleic acid parallel testing liquid-phase chip as well as preparation method and application thereof
A parallel detection and liquid phase chip technology, which is applied in the field of molecular biology technology and clinical detection, can solve the problems of waste of drug resources, high detection costs, bacterial drug resistance, etc., and achieve the effect of saving detection costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Example 1: Coupling of probes to known numbered microspheres
[0060] (1) Select 11 kinds of different fluorescent colors (microspheres 11, 15, 22, 28, 36, 42, 49, 55, 56, 71, and 75) carboxylated microspheres (Luminex company), and oscillate with a vortex shaker Microsphere suspension, the time is 20s, so that the microspheres are mixed evenly.
[0061] (2) Take about 2×10 carboxyl microspheres of the above numbers respectively. 3 Transfer each to a centrifuge tube and centrifuge at ≥8000×g for 2 min to precipitate carboxylated microspheres.
[0062] (3) Remove the supernatant, add 100 μ ldH 2 O, use a vortex shaker to resuspend the microspheres for 20s, centrifuge at ≥8000×g for 2min, and precipitate the carboxylated microspheres.
[0063] (4) Remove the supernatant, add 80 μl, 100 mM, pH = 6.2 sodium dihydrogen phosphate salt solution, and resuspend the washed carboxyl microspheres with a vortex shaker for 20 s.
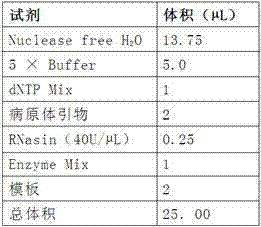
[0064] (5) Add 10μl, 50mg / ml Sulfo-NHS (with dH ...
Embodiment 2
[0076] Example 2: The application of the parallel detection liquid phase chip for respiratory tract infection pathogens of the present invention in clinical detection
[0077] The technical process of using the liquid chip for parallel detection of respiratory tract infection pathogens of the present invention to detect respiratory tract infection pathogens:
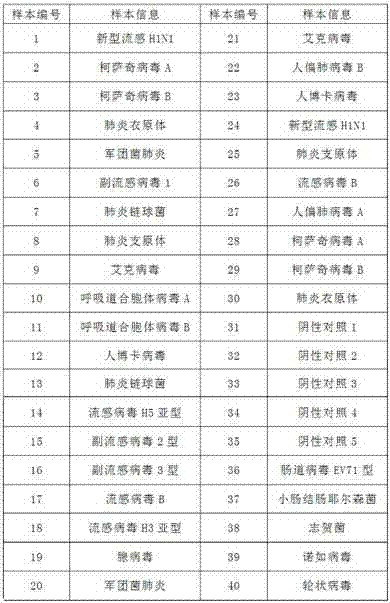
[0078] 1. Extraction of pathogen nucleic acid: RNA can be extracted using commercial kits, such as QIGEN Viral RNA mini Extraction Kit (CAT: 52904) to extract pathogen RNA from clinical specimens or pathogen isolation cultures; a total of 40 specimen nucleic acids were extracted, Of these, 30 were positive samples with known subtypes, 5 were negative controls, and 5 were samples of unrelated pathogens. According to the experimental requirements, another blank control was set up. Specimen information is as follows:
[0079]
[0080] (1) Add 560 μl of AVL buffer to a 1.5 ml centrifuge tube;
[0081] (2) Add 140 μl cl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com