Liquid phase chip for parallel detection of autoantibodies, preparation method and application thereof
A parallel detection, autoantibody technology, applied in the field of immune technology and clinical detection, can solve problems such as inconvenience and achieve the effect of saving detection costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1: Coupling of self-antigens to known numbered microspheres
[0024] 1. Select No. 28, 38, 48, 58, 68, and 78 carboxyl microspheres (Luminex Company) respectively, and oscillate the microsphere suspension with a vortex shaker for 20 seconds to mix the microspheres evenly.
[0025] 2. Take about 2×10 carboxyl microspheres of each number above. 3 Transfer each to a centrifuge tube and centrifuge at ≥8000×g for 2 min to precipitate carboxylated microspheres.
[0026] 3. Remove the supernatant and add 100μl dH 2 O, use a vortex shaker to resuspend the microspheres for 20 seconds, centrifuge at ≥8000×g for 2 minutes, and precipitate the carboxylated microspheres.
[0027]4. Remove the supernatant, add 80 μl, 100 mM, pH=6.2 sodium dihydrogen phosphate salt solution, and resuspend the washed carboxy microspheres with a vortex shaker for 20 seconds.
[0028] 5. Add 10μl, 50mg / ml Sulfo-NHS (with dH 2 O dilution), oscillate gently with a vortex shaker.
[0029] 6. Ad...
Embodiment 2
[0040] Example 2: Application of liquid phase chip for parallel detection of autoimmune diseases described in the present invention in clinical detection
[0041] (1) The technical process of using the autoantibody parallel detection liquid phase chip of the present invention to detect autoantibodies:
[0042] 1. Take out 500 microspheres conjugated to each autoantigen prepared above, mix them in equal proportions, and distribute them in 96-well plates. Each well contains 500 microspheres conjugated to various autoantibodies. Add 50 μl of serum to be tested and incubate at 37°C for 2 hours.
[0043] 2. Centrifuge at ≥8000×g for 2 minutes, remove the supernatant, add 300 μl of 1% PBS-BSA, vortex for 30 seconds, and seal in a 37°C incubator for 1 hour.
[0044] 3. Centrifuge at ≥8000×g for 2 minutes. Remove the supernatant, add 300 μl PBS-TBN, and vortex for 30 seconds. Centrifuge at ≥8000×g for 2 minutes.
[0045] 4. Repeat step 3 twice.
[0046] 5. Add 100 μl of the bioti...
Embodiment 3
[0055] Example 3: Example 1 of standard curve drawing of autoantibodies
[0056] According to the method described in Examples 1 and 2, SSA / Ro-52 (R052) was coated onto No. 38 microspheres. After coating, 1000 microspheres were added to PE-labeled mouse anti-human IgG to react for 30 minutes, and detected by Luminex100 , the fluorescence value reached 23,000, while the microspheres not coated with SSA / Ro-52 were reacted with PE-labeled mouse anti-human IgG for 30 minutes. After Luminex100 detection, the fluorescence value was only 100, indicating that our coated microspheres can meet the needs of chip development. requirements.
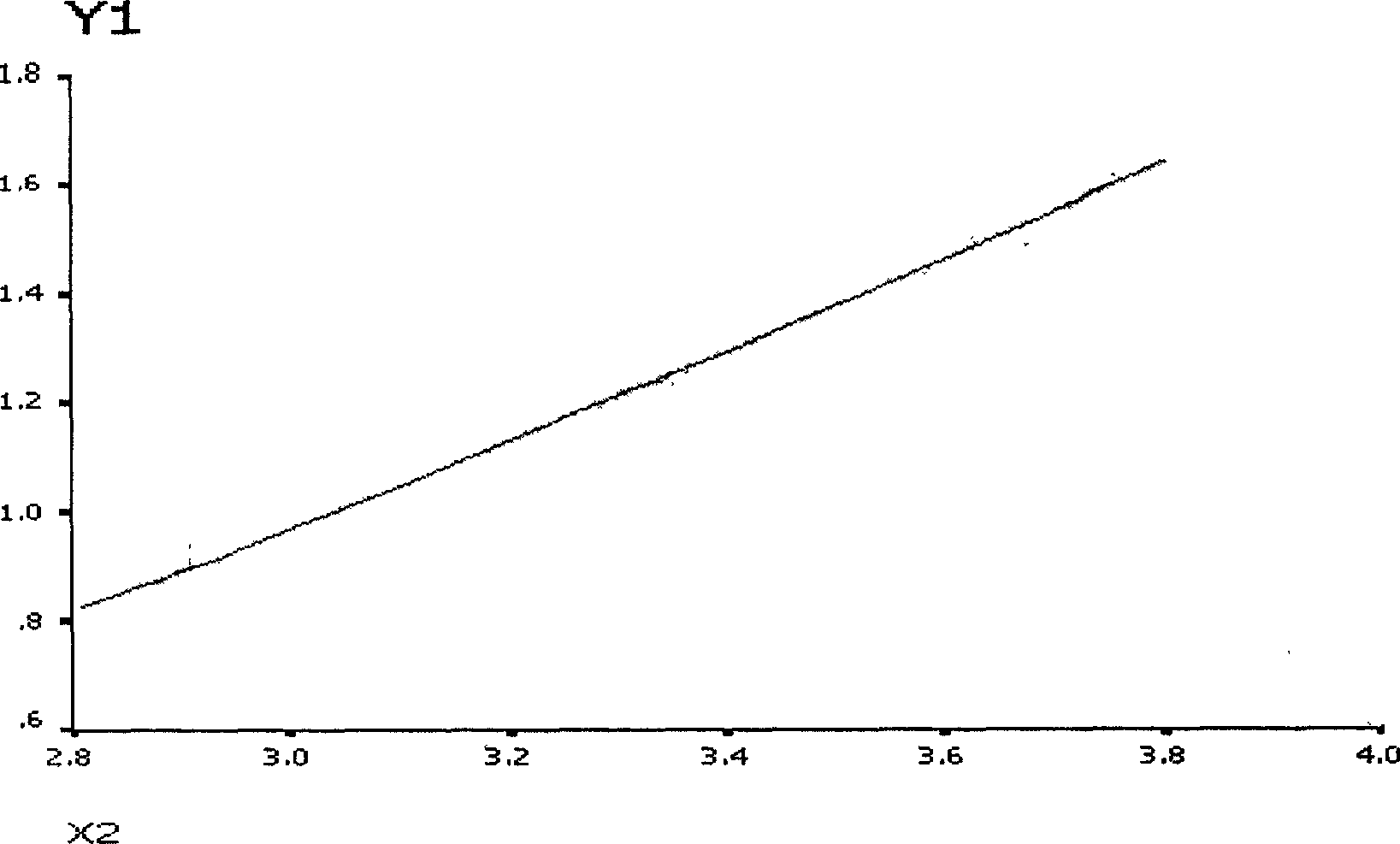
[0057] Take the standard SSA / Ro-52 antibody, dilute it according to the gradient of 0EU / ml, 6.25EU / ml, 12.5EU / ml, 25EU / ml and 50EU / ml, react according to the method described, and detect by Luminex100, the average fluorescence intensity is respectively for 261.5, 908, 1475, 4422 and 6654.5. For the standard curve obtained, see figure 1 . One case ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| fluorescence | aaaaa | aaaaa |
| fluorescence | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com