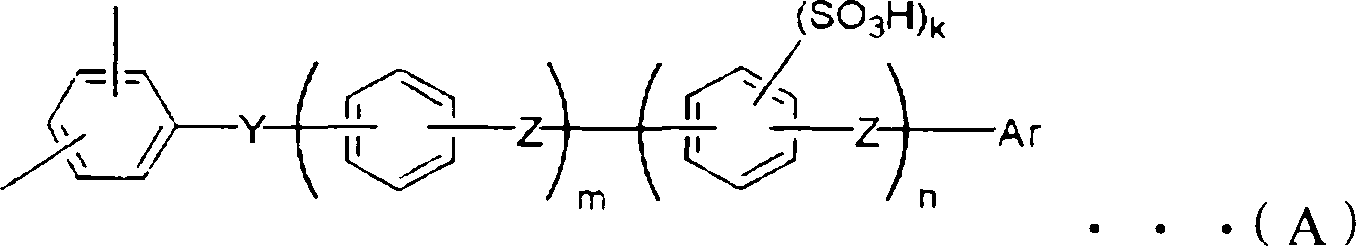
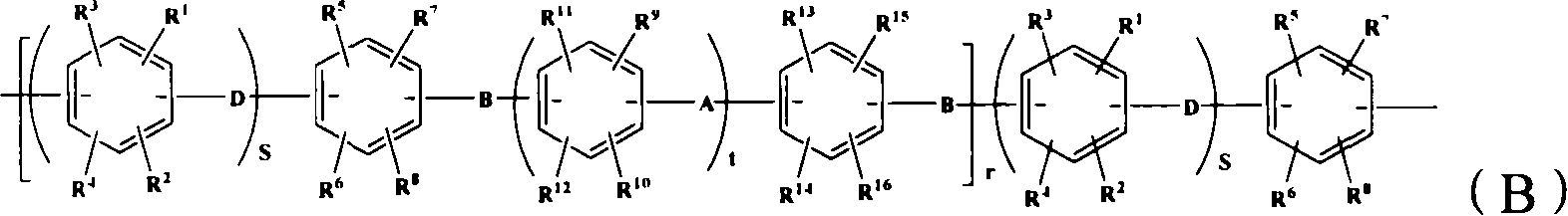
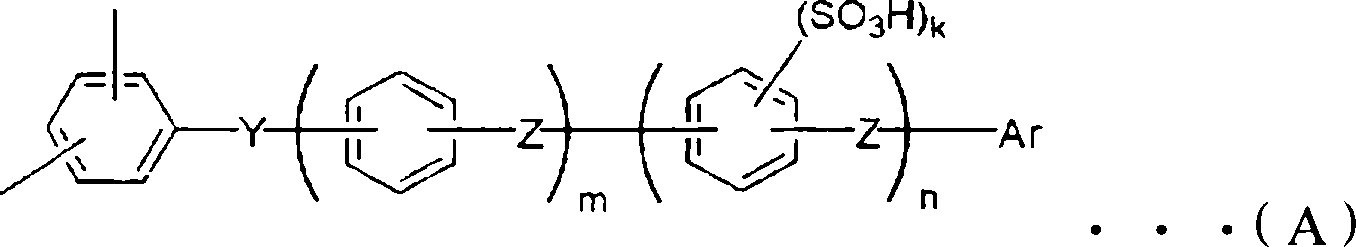
Proton conduction film
A proton-conducting membrane and ion-conducting technology, applied in the field of proton-conducting membranes, can solve problems such as ion conductivity decline
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example 1
[0181] (modulation of oligomers)
[0182] Add 67.3g (0.20 moles) of 2,2-bis(4-hydrobenzene)-1,1 to a 1L three-necked Erlenmeyer flask equipped with a stirrer, a thermometer, a cooling pipe, a Dean-Stark pipe, and a three-way valve for introducing nitrogen, 1,3,3,3-hexafluoropropane, 60.3g (0.24 mol) of 4,4'-dichlorobenzophenone (4,4'-DCBP), 71.9g (0.52 mol) of potassium carbonate, 300mL of N , N-dimethylacetamide (DMAc), and 150 mL of toluene were heated and stirred in an oil bath under a nitrogen atmosphere, and the reaction was carried out at 130° C. When the water and toluene generated by the reaction were azeotropically removed from the reaction system through the Dean-Stark tube to carry out the reaction, it was confirmed that almost no water was generated after about 3 hours. The reaction temperature was slowly increased from 130 to 150°C. Then, in the process of slowly increasing the reaction temperature to 150°C, remove most of the toluene, and continue the reaction ...
Synthetic example 2
[0188] Modulation of Polyarene Polymer Using Neopentyl as Protecting Group
[0189] Add 39.58g (98.64mmol) of 3-(2,5-Di Neopentyl benzenesulfonate, and 15.23 g (1.36 mmol) of the BCPAF oligomer (Mn=11200) prepared in Synthesis Example 1, 1.67 g (2.55 mmol) of Ni(PPh 3 ) 2 Cl 2 , 10.49g (40 mmol) of PPh 3 , 0.45 g (3 mmol) of NaI, 15.69 g (240 mmol) of zinc powder, 390 mL of dry NMP. Under the condition of stirring the reaction system, it was heated (finally heated to 75° C.) for 3 hours. Dilute the polymerization reaction liquid with 250 mL of THF, stir for 30 minutes, use celite as a filter aid, filter, and pour the filtered liquid into excess 1500 mL of methanol to make it solidify. The coagulum was collected by filtration, air-dried and redissolved in THF / NMP (200 / 300mL, respectively), and coagulated and precipitated with an excess of 1500mL methanol. After air-drying, 47.0 g (recovery rate 99%) of the desired yellow fibrous, neopentyl-protected, sulfone derivative co...
Synthetic example 3
[0195] (synthesis of oligomers)
[0196]Use 103.7g (0.48mol) of 4,4'-dihydrobenzophenone (4,4'-DHBP), 148.2g (0.52mol) of 4,4'-dichlorodiphenylsulfone (4,4'-DCDS ), 86.9g (0.63mol) of potassium carbonate, 500mL of 1,3-dimethyl-2-imidazolethane (DMI), 200mL of toluene, and the same steps as in Synthesis Example 1 to carry out the reaction operation to obtain the target compound 180g ( Recovery rate 78%).
[0197] The number average molecular weight of the obtained polymer was 13,700 in terms of polyethylene as determined by GPC (THF solvent). In addition, the obtained polymer is soluble in NMP, DMAc, DMI, etc., has a Tg of 159°C, and a thermal decomposition temperature of 500°C.
[0198] The obtained compound is presumed to be an oligomer having a structure represented by formula (III) (hereinafter will be referred to as "C oligomer").
[0199] "Chemical 23"
[0200]
PUM
| Property | Measurement | Unit |
|---|---|---|
| Viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com