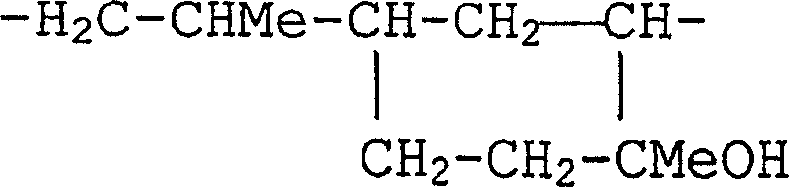Cross-linkable products based on organosilicon compounds
A technology of organosilicon compound and cross-linking composition, which is applied in the direction of filling slurry, etc., and can solve the problems of short biocide activity time, discoloration, chemical instability, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0168] Manufacture of polyquaternary polysiloxanes:
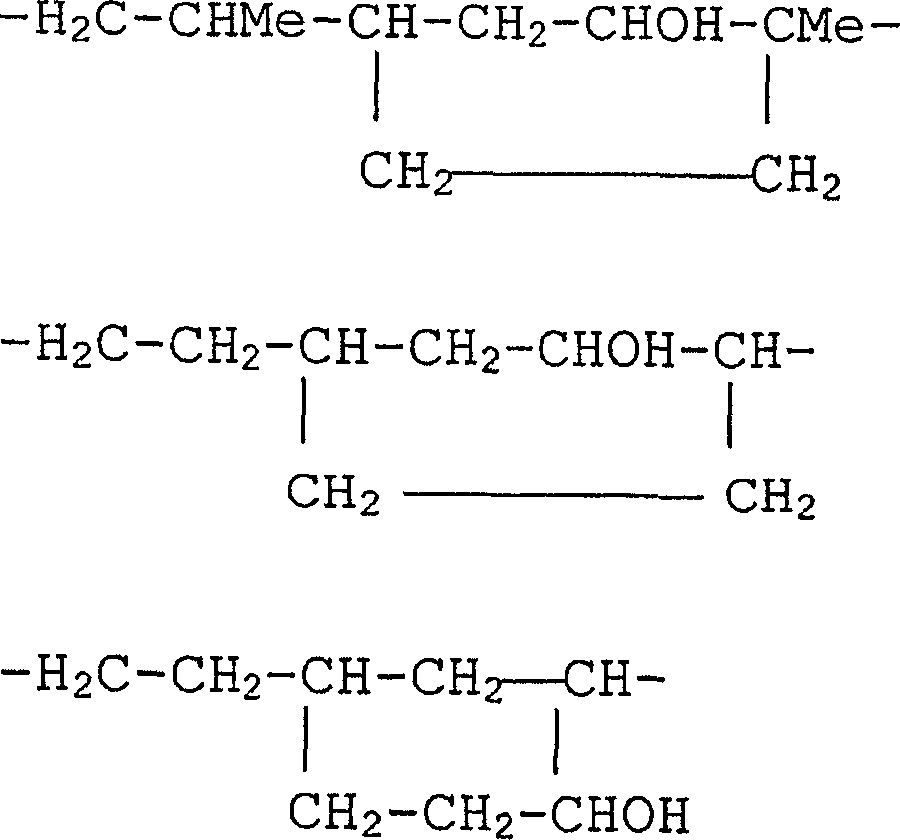
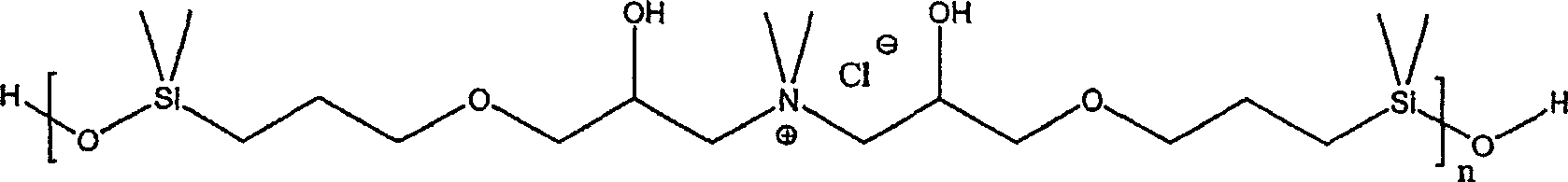
[0169] Dissolve 286.4 g of dimethylammonium chloride in 1000 ml of water, add 1200 g of 1,3-bis(3-glycidoxypropyl)-1,1,3,3-tetramethyldisiloxane , and the mixture was refluxed with vigorous stirring. The reaction mixture was stirred at 105 to 110°C for 2 hours, where the batch changed from colorless turbidity to clear yellow. The solvent was removed in vacuo at 120°C. The reaction product is a viscosity of about 16×10 6 A dark yellow, highly viscous oil in mPa·s. 1 H-NMR spectral analysis indicated the formation of polyquaternary polysiloxanes having an average of 18 to 20 repeating units corresponding to the formula below.
[0170]
[0171] In a planetary mixer with vacuum equipment, under anhydrous conditions, 1400 grams of -OSi(OCH 3 ) 2 (CH 3 ) terminal group, polydimethylsiloxane with a viscosity of 80,000 mPa·s and 600 grams of -OSi(CH 3 ) 3 Polydimethylsiloxane terminated with a viscosity of 100 mPa·s, 12...
Embodiment 2
[0177] 1400 g of α,ω-dihydroxypolydimethylsiloxane with a viscosity of 80,000 mPa·s, 12 g of polyquaternary polysiloxane produced as described in Example 1 were mixed under vacuum in a planetary mixer. Oxane, 300 grams have -OSi (CH 3 ) 3Polydimethylsiloxane with a terminal group and a viscosity of 100 mPa·s, a dynamic viscosity of 6.2 mm2 / s (at 40°C) for 300 g, a viscosity density constant (VDK) of 0.79, and a boiling range of 300°C to 370°C (carbon distribution: 62% paraffin carbon atoms, 38% cycloalkane carbon atoms and 0.03% aromatic hydrocarbon carbon atoms) hydrocarbon mixture, 90 grams of ethyl triacetoxysilane and 190 grams of specific surface area of 150 square meters / grams of fumed hydrophilic silica to be uniformly mixed. Then 0.5 g of dibutyltin diacetate were added and homogenization was carried out for a further 5 minutes.
[0178] Test specimens as described in Example 1 were prepared from the compositions thus obtained and tested according to DIN ENI ISO...
Embodiment 3
[0181] The procedure described in Example 1 was repeated except that the amount of polyquaternary polysiloxane was doubled.
[0182] Test specimens as described in Example 1 were prepared from the compositions thus obtained and tested according to DIN ENI ISO 846. The obtained results are shown in Table 1.
[0183] To determine storage stability, the samples were stored at 100° C. for 3 days in air-moisture-tight containers. There was no change in the curing characteristics or appearance of the samples, which indicates outstanding storage stability and resistance to yellowing.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com