New crystalline type 3,4',5-trihydroxy stilbene-3-beta-D-glucocide
A technology of glucoside and trihydroxystilbene is applied in the field of 3,4',5-trihydroxystilbene-3-β-D-glucoside of new crystal form, which can solve the problems of double melting point and long melting range, and achieve Good compressibility, reduced raw material cost and easy quality control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] [Example 1] Preparation of type II crystal form 3,4',5-trihydroxystilbene-3-β-D-glucoside
[0034] 3,4',5-trihydroxystilbene-3-β-D-glucoside (polydatin) raw material compound can be prepared by the method described in Chinese patent application 03117246; solvent ethanol and acetone are respectively re-distilled to obtain refined ethanol and refined acetone . Add 300g of polydatin to about 1L of refined ethanol solvent, dissolve and filter, concentrate the filtrate under reduced pressure (0.1Mpa, 55°C) to about 0.5L, add about 1L of refined acetone while it is hot, and place the mixed solution in a refrigerator at 4°C to precipitate a solid The product was vacuum-dried (0.1Mpa, room temperature) after suction filtration to obtain about 226g of type II polydatin.
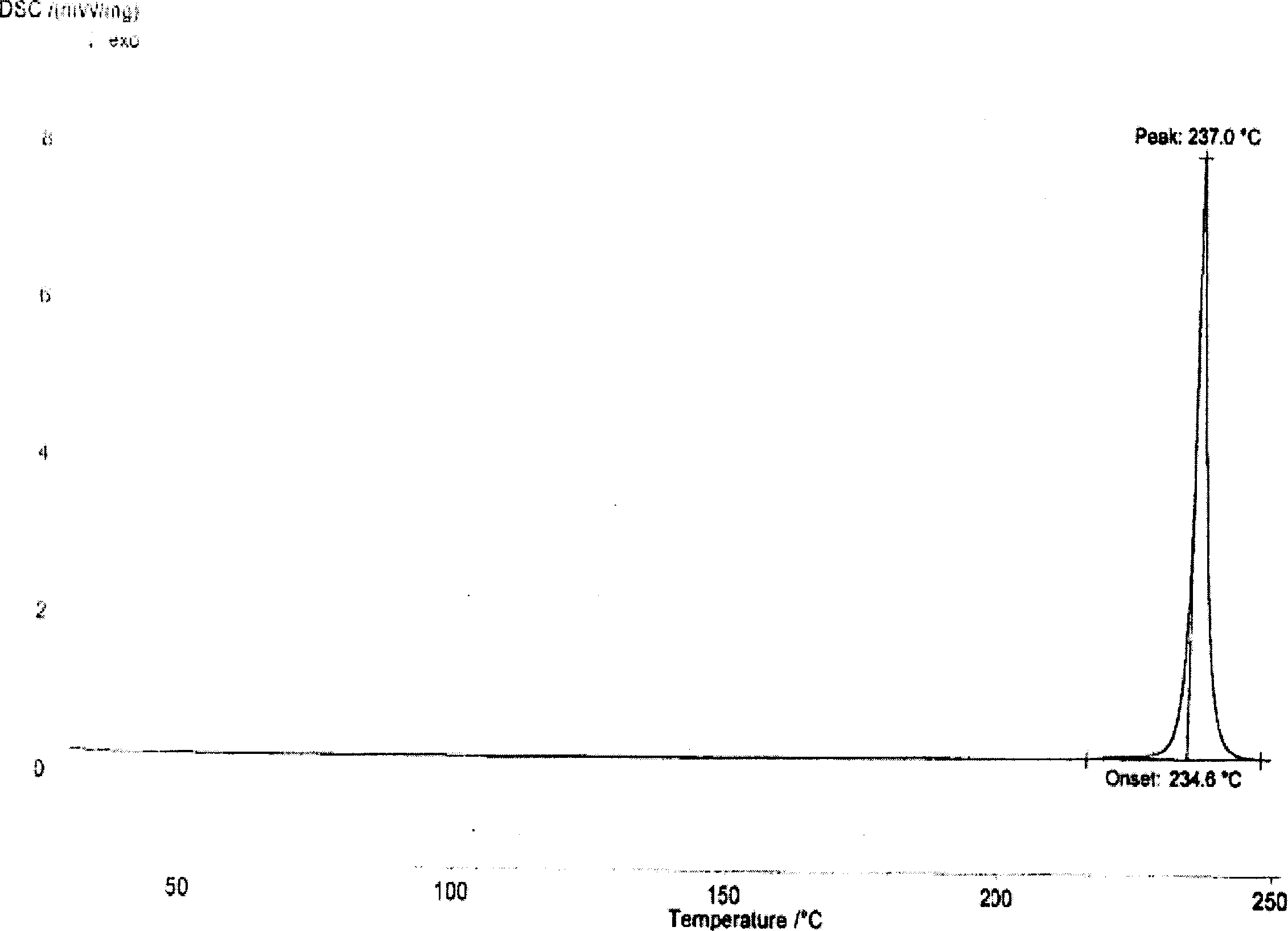
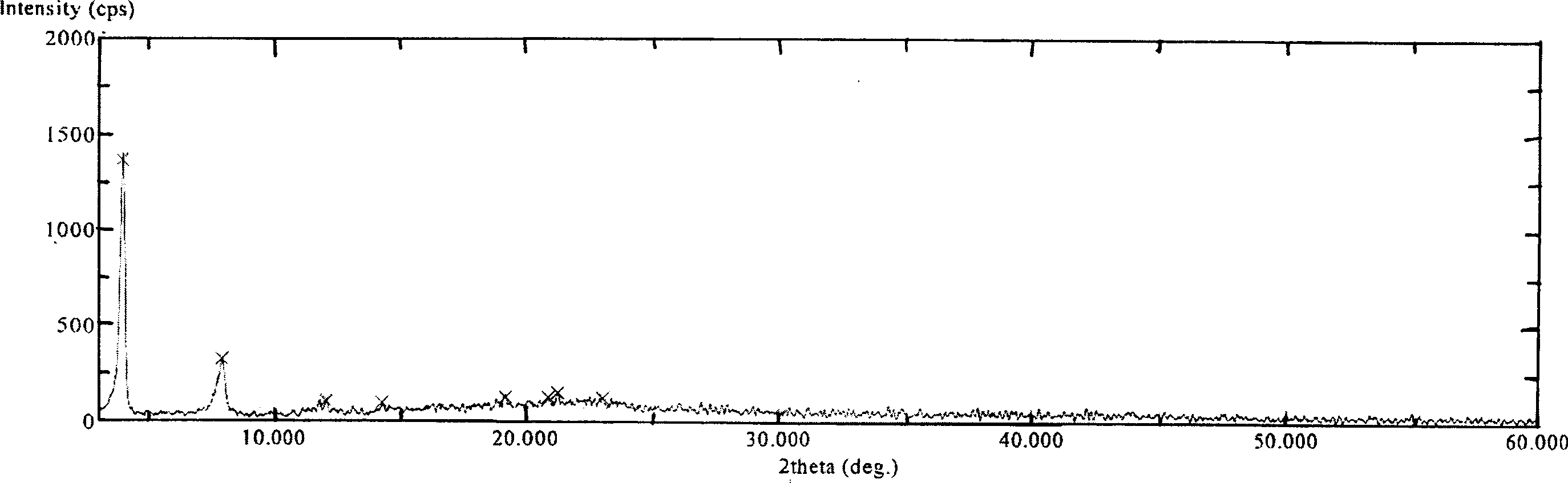
[0035] Its XRD spectrum is as figure 1 As shown, the DSC spectrum is as figure 2 shown.
Embodiment 2
[0036] [Example 2] Preparation of Type III crystal form 3,4',5-trihydroxystilbene-3-β-D-glucoside
[0037] 3,4', 5-trihydroxystilbene-3-β-D-glucoside (polydatin) raw material compound can be prepared by the method described in Chinese patent application 03117246; solvent acetone, ethyl acetate, n-hexane are refined respectively to obtain refined Acetone, ethyl acetate and refined n-ethane. Add 100g of polydatin into about 1.5L of refined acetone solvent, dissolve and filter, concentrate the filtrate under reduced pressure (0.1Mpa, 55°C) to about 0.8L, add about 0.3L of refined ethyl acetate while it is hot, mix well, add Refined about 1 L of n-hexane, and placed the mixed solution in a refrigerator at 4°C. The precipitated solid was suction filtered and then vacuum-dried (0.1 Mpa, room temperature) to obtain about 69 g of type III polydatin.
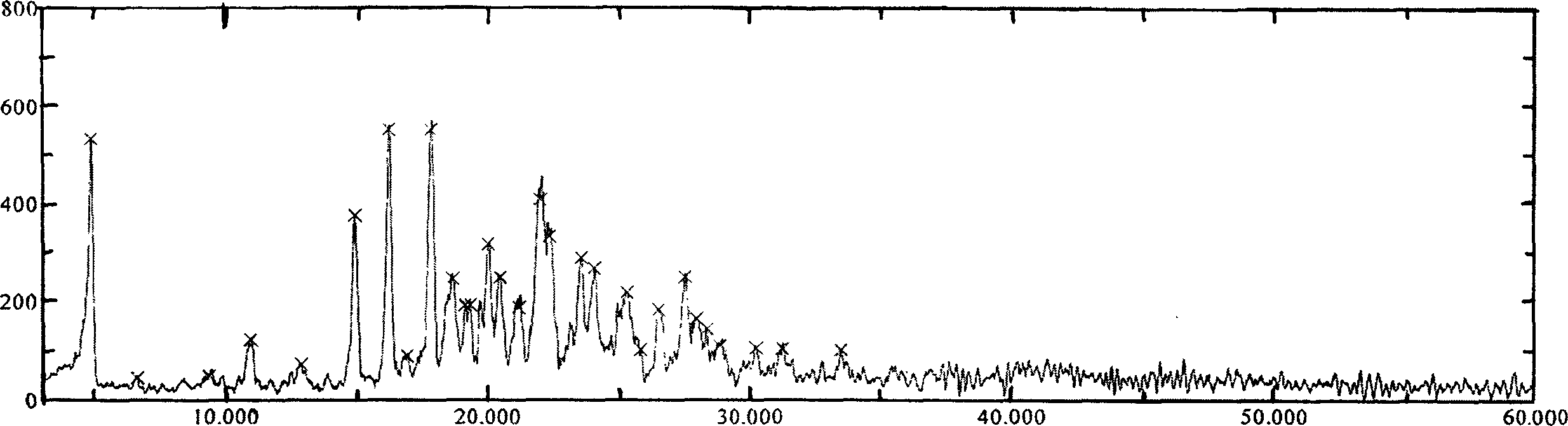
[0038] Its XRD spectrum is as image 3 As shown, the DSC spectrum is as Figure 4 shown.
[0039] 0.5g of 3,4',5-trihydroxystilbene...
Embodiment 3
[0040] [embodiment 3] the preparation of solid pharmaceutical preparation
[0041] Element
[0042] Preparation method: The above prescription ingredients are mixed according to conventional preparation methods, and directly compressed into tablets.
[0043] Element
[0044] Preparation method: Type II crystal polydatin (Example 1) is mixed evenly with starch, lactose, and dextrin according to the method of equal multiplication, and the pre-prepared HPMC solution is added to make a soft material, granulated with a 20-mesh sieve, and dried at 60°C for about After 30 minutes, sieve with 18 meshes, add micropowder silica gel, mix evenly, and fill with 2# capsules.
[0045] Element
[0046] Preparation method: The above prescription ingredients are mixed, granulated and compressed into tablets according to conventional preparation methods.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com