Levamlodipine besylate crystal and preparation method and application thereof
A technology for levamlodipine besylate and crystals, applied in the field of preparation thereof, and crystals of levamlodipine besylate, can solve the problems such as the lack of clear crystallographic main parameter atomic space positions, the lack of clear crystal water molecules and the like , to achieve the effect of being conducive to stability, improving solubility and stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Embodiment 1 Preparation of levamlodipine besylate crystal of the present invention
[0049] 1) Completely dissolve 5 grams of levamlodipine besylate powder sample (provided by Shihuida Pharmaceutical Group (Jilin) Co., Ltd., batch number: 140207-2) into 2.5 mL of a mixed solution of distilled water and acetone with a volume ratio of 1:1 to obtain the reaction solution;
[0050] 2) Raise the temperature of the reaction solution to 30°C, stir for 10 minutes, cool to room temperature, then slowly add 10 mL of distilled water, let it stand, and lower the ambient temperature to 5°C, keep this condition for 1 day, and obtain a large number of needle-shaped or rod-shaped Color transparent crystal, regular crystal form, uniform particle size;
[0051] 3) Filter the obtained crystal product under reduced pressure, wash the original crystal solution, and wash with cold water.
specific Embodiment 2-7
[0052] Following specific examples 2-7, preparation method is the same as embodiment 1, and its concrete process parameter is shown in table 1.
[0053] Table 1
[0054]
[0055]
Embodiment 8
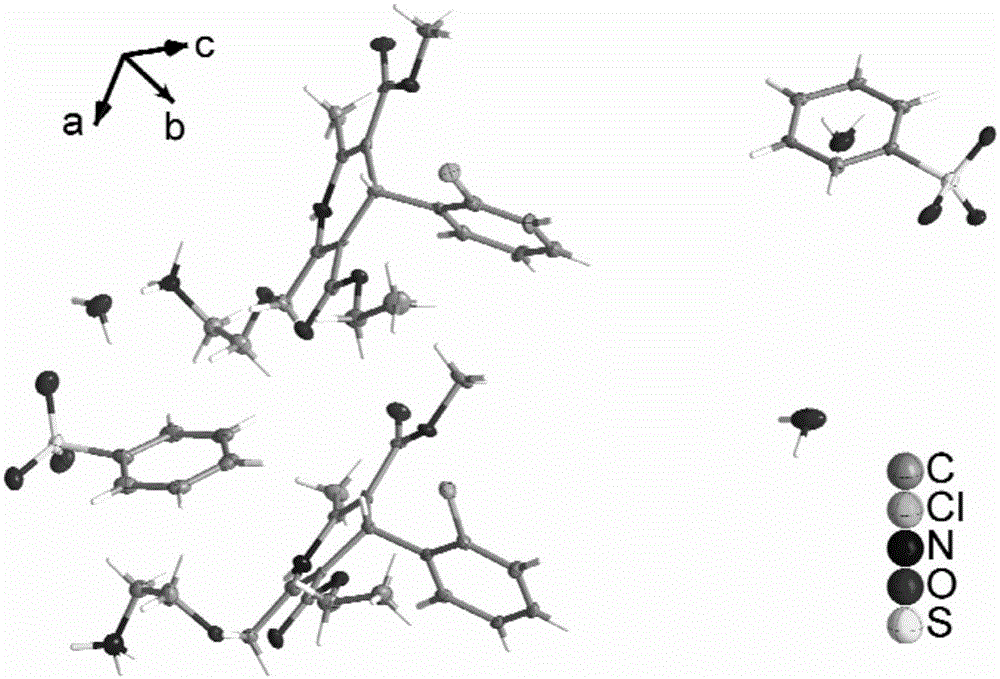
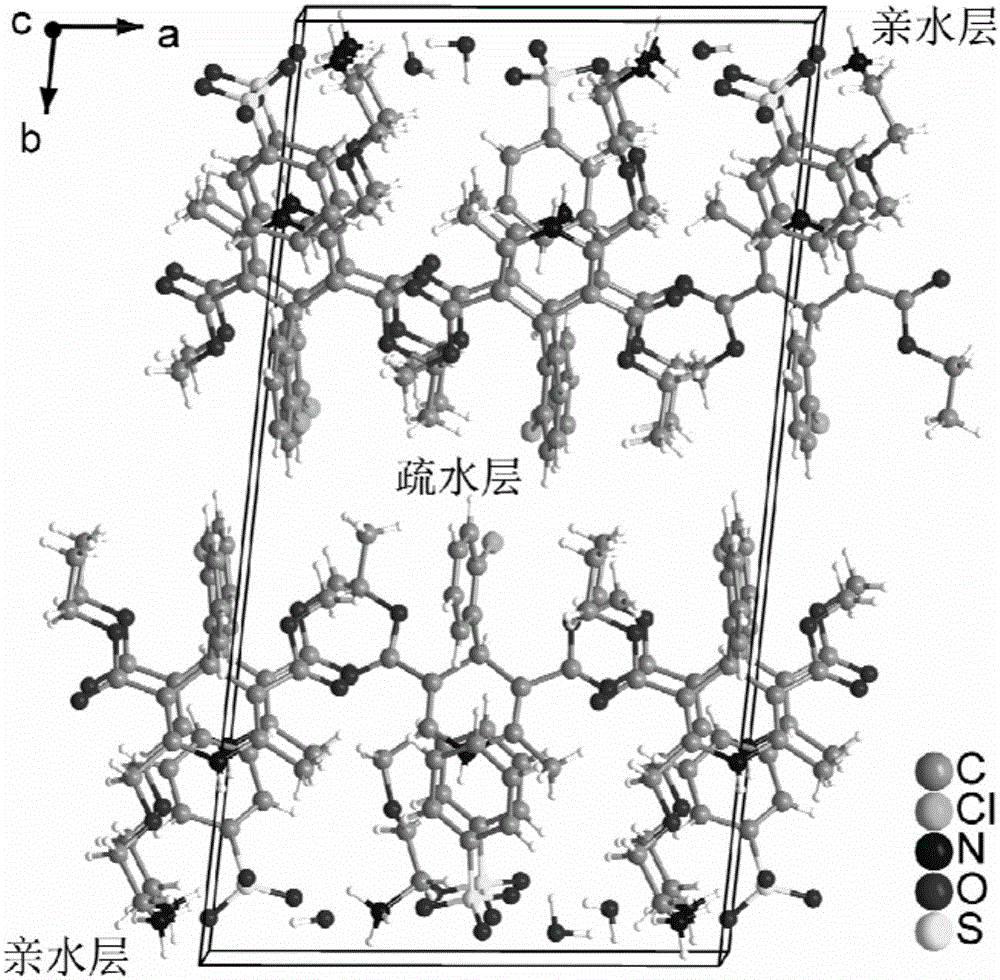
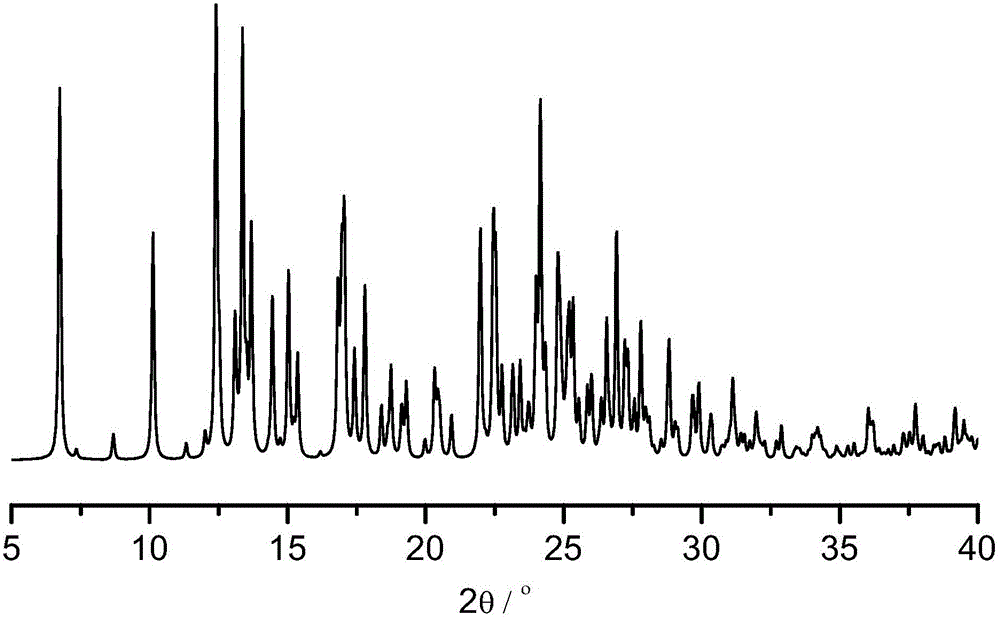
[0056] The measurement of the crystal form of embodiment 8 levamlodipine besylate crystals
[0057] For each of the above examples, select a crystal with a size of 0.6×0.8×2.1 (mm) from the prepared crystal sample, and "rivet" it on the top of the glass filament, and adopt an X-ray single crystal diffractometer of the ApexDuo model of Bruker, Germany The test is carried out, and the test parameters are carried out according to the strategy established by the instrument according to the crystal size. The test temperature is 173K, with MoK α radiation Diffraction data were collected in ω-scan mode and Lp correction was performed. Absorption correction was performed using the SADABS program. Use the direct method to analyze the structure, use the difference Fourier method to find all non-hydrogen atoms, all hydrogen atoms on carbon and nitrogen are obtained by theoretical hydrogenation, and the hydrogen atoms of crystal water molecules are directly found from the difference F...
PUM
| Property | Measurement | Unit |
|---|---|---|
| solubility (mass) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com