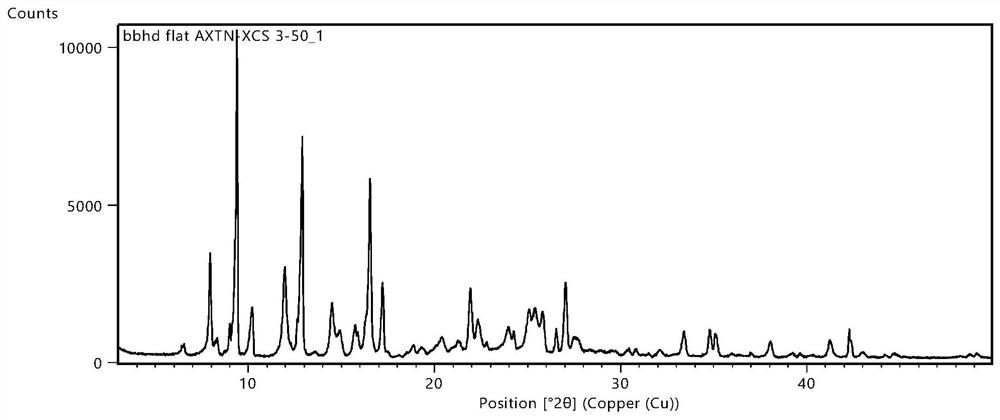
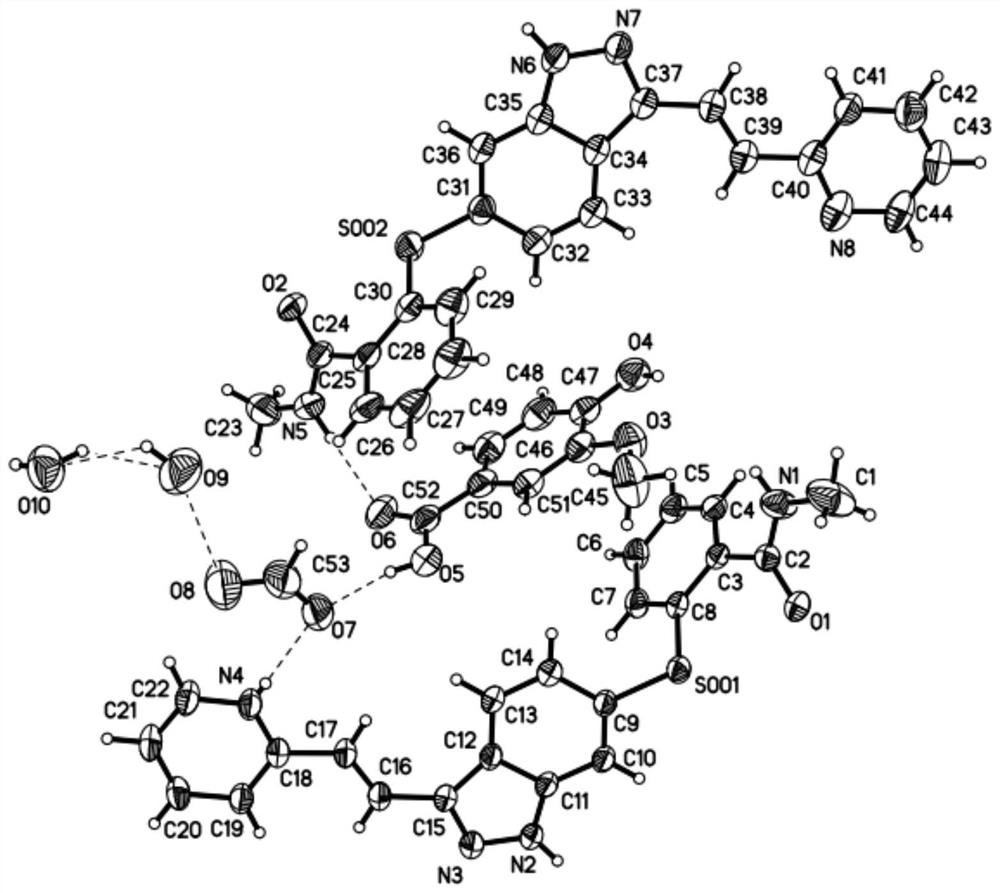
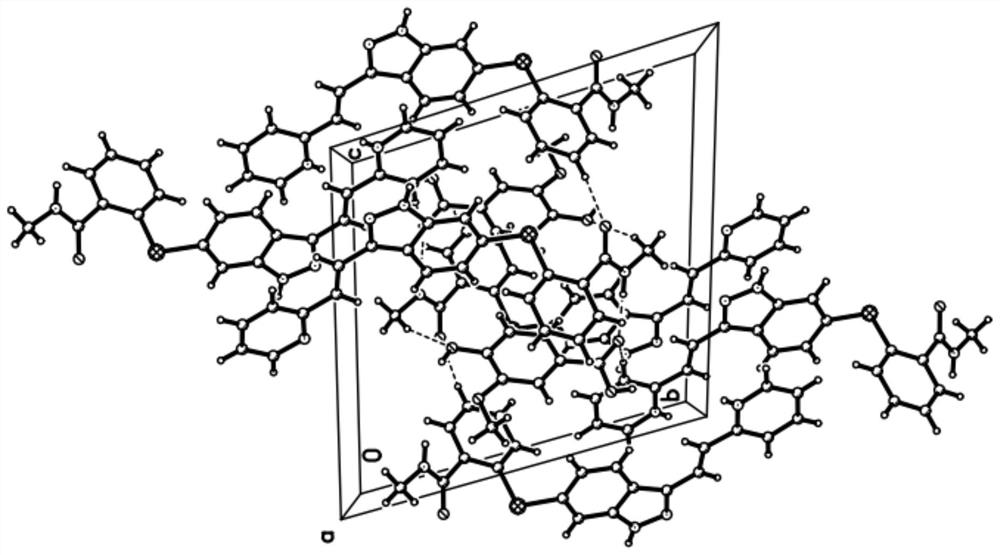
Axitinib vanillic acid eutectic salt and preparation thereof
A technology of axitinib and co-, axitinib, applied in the direction of carboxylate preparation, medical preparations containing active ingredients, separation/purification of carboxylic acid compounds, etc., can solve the problem of inability to judge the mode of action of API and CCF, No problems such as the structural information of the resveratrol complex are given, and the effects of good photostability, regular crystal form, and uniform particle size are achieved
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Add about 0.8g axitinib, 0.27g vanillic acid to 120ml ethanol / formic acid / water (V 乙醇 :V 甲酸 :V 水 =5:2:1) in the mixed solution, heated to 65-70 °C to dissolve, stirred and refluxed for 5 hours, cooled to 10-15 °C, temperature-controlled crystallization, crystallization was completed, filtered, the filter cake was washed with ethanol, dried, Axitinib-vanillic acid co-crystal salt was obtained, with a purity of 99.55%.
Embodiment 2
[0045] Add about 0.8g axitinib and 0.17g vanillic acid to 100ml methanol / formic acid / water (V 甲醇 :V 甲酸 :V 水 =1:1:1) in the mixed solution, heated to 60~65℃ to dissolve, stirred and refluxed for 6 hours, then cooled to 15~20℃, temperature controlled for crystallization, crystallization was completed, filtered, the filter cake was washed with methanol, dried, Axitinib-vanillic acid co-crystal salt was obtained, with a purity of 99.52%.
Embodiment 3
[0047] Add about 0.8g axitinib, 0.34g vanillic acid to 160ml isopropanol / formic acid / water (V 异丙醇 :V 甲酸 :V 水 =10:3:1) in the mixed solution, heated to 60~70 ℃ to dissolve, stirred and refluxed for 3 hours, cooled to 20~30 ℃, temperature controlled crystallization, crystallization was completed, filtered, and the filter cake was washed with isopropanol, After drying, axitinib-vanillic acid co-crystal salt was obtained with a purity of 99.48%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com