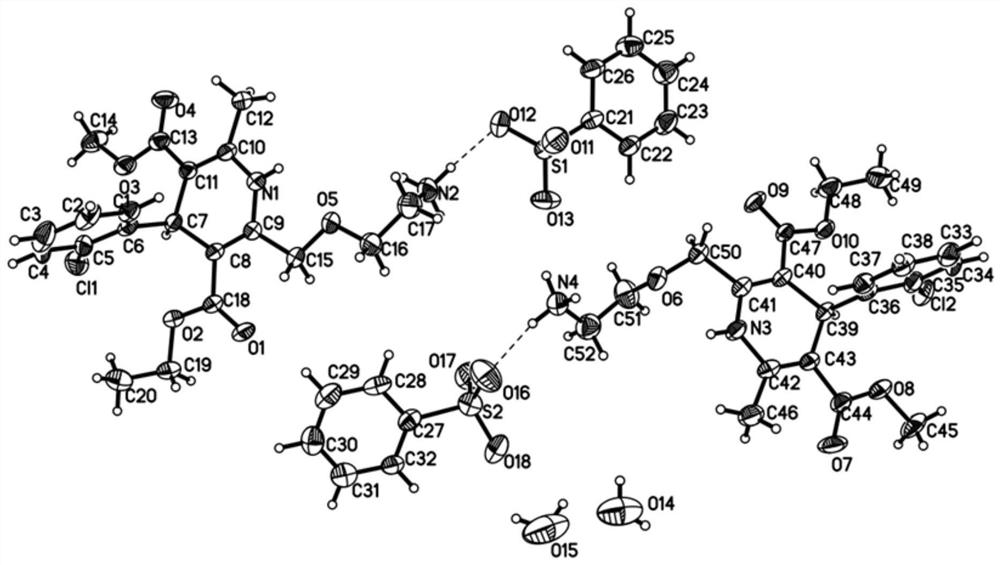
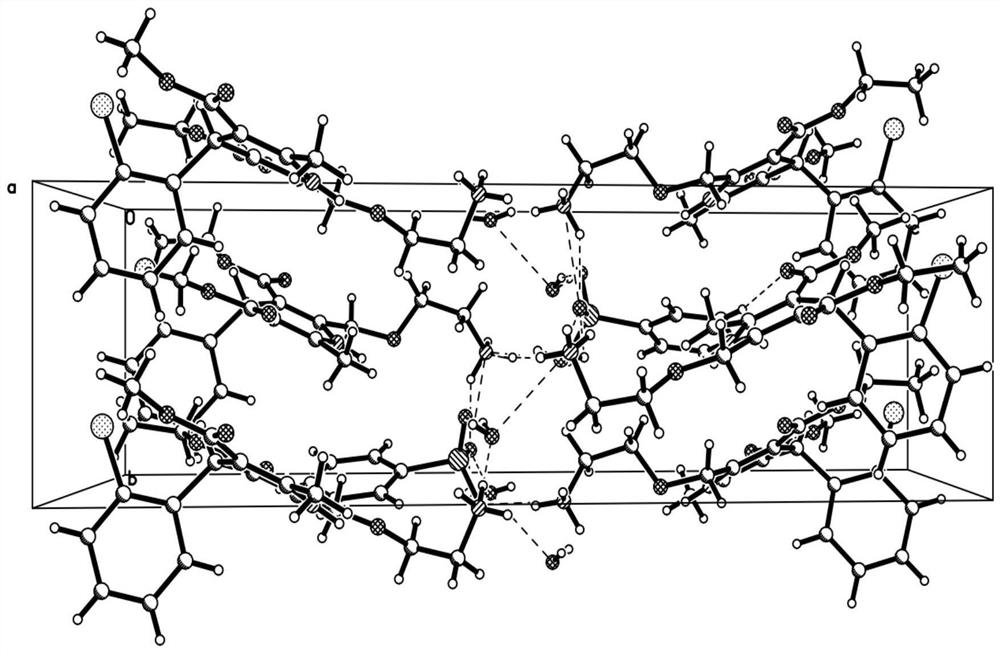
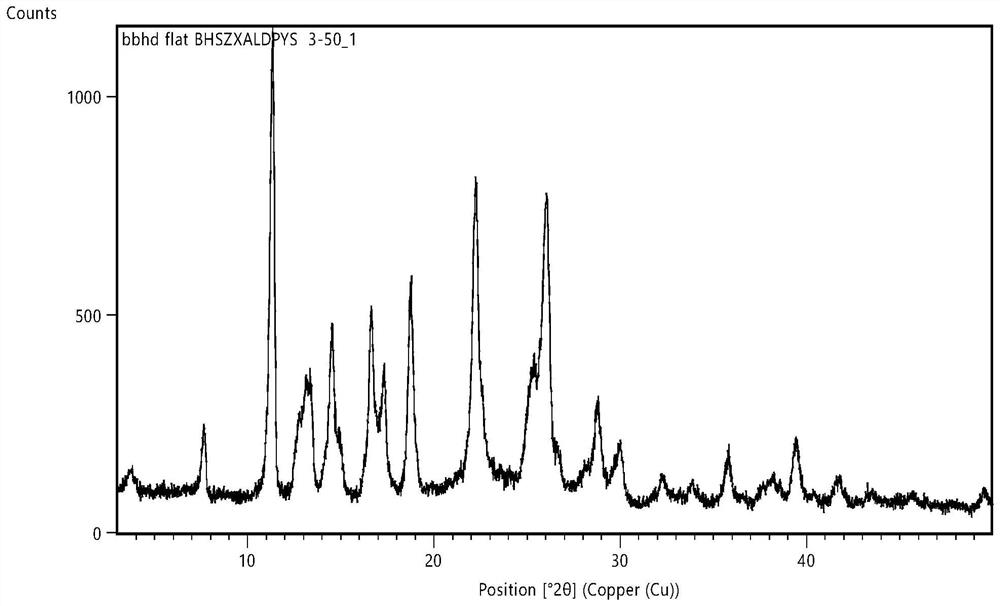
New crystal form of levamlodipine besylate
A kind of technology of levamlodipine besylate and levamlodipine besylate, applied in the field of levamlodipine besylate crystal form, can solve the problem that solubility, thermal stability, light stability, dissolution and bioavailability are not very good Meet the requirements of pharmaceutical preparations and other issues, and achieve the effects of increasing bioavailability, good stability, and simple preparation methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Add levamlodipine besylate (5.05g) into ethanol / purified water (V 乙醇 :V 水 =1:8, 450ml) in the mixed solution, heated to 50°C and stirred to dissolve, continued to insulate and stir for 0.5h, then filtered, and the filtrate was lowered to room temperature at a rate of 0.5°C / min; the filtrate was placed in a beaker, sealed The membrane was sealed, punctured, volatilized, crystallized for 3 days, filtered, and dried under reduced pressure at 45°C to constant weight to obtain the crystalline form of L-amlodipine besylate with a yield of 98.2%, an optical purity of 99.97%, and a purity of 99.92%.
Embodiment 2
[0043] Add levamlodipine besylate (5.03g) into acetone / purified water (V 丙酮 :V 水 =1:6, 400ml) in the mixed solution, heated to 40°C and stirred to dissolve, continued to insulate and stir for 1 hour, then filtered, and the filtrate was cooled to room temperature at a rate of 0.5°C / min; the filtrate was placed in a beaker, sealed with Seal, pierce, volatilize, crystallize for 2 days, filter, and dry under reduced pressure at 45°C to constant weight to obtain the crystalline form of L-amlodipine besylate with a yield of 97.7%, an optical purity of 99.95%, and a purity of 99.82%.
Embodiment 3
[0045] Levoamlodipine besylate (5.02g) was added to methanol / purified water (V 甲醇 :V 水 =1:7, 450ml) in the mixed solution, heated to 50°C and stirred to dissolve, continued to insulate and stir for 0.5h, then filtered, and the filtrate was cooled to room temperature at a rate of 0.5°C / min; the filtrate was placed in a beaker, sealed The membrane was sealed, punctured, volatilized, crystallized for 3 days, filtered, and dried under reduced pressure at 45°C to constant weight to obtain the crystalline form of L-amlodipine besylate with a yield of 97.5%, an optical purity of 99.93%, and a purity of 99.84%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com