Carbon-based fuel cell
A fuel cell, organic fuel technology, used in fuel cells, battery electrodes, circuits, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
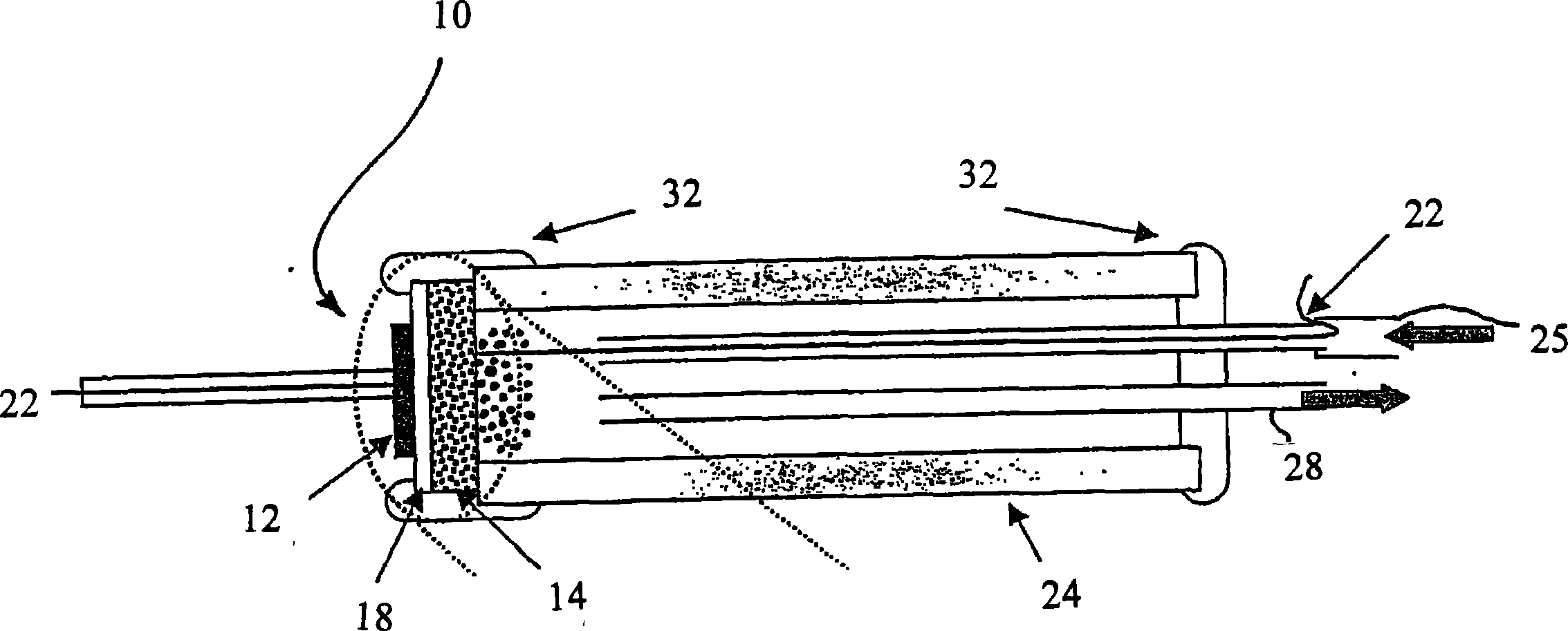
[0044] exist image 3 In , a test assembly comprising a fuel cell 10 of the present invention is shown. The fuel cell 10 consists of a dense disc of YSZ (from Tosoh Corp.) coated with a thin layer of anode and cathode catalyst material to form the electrodes 12,14. Detailed steps involved in catalyst preparation and fuel cell 10 construction are explained in Example 2.
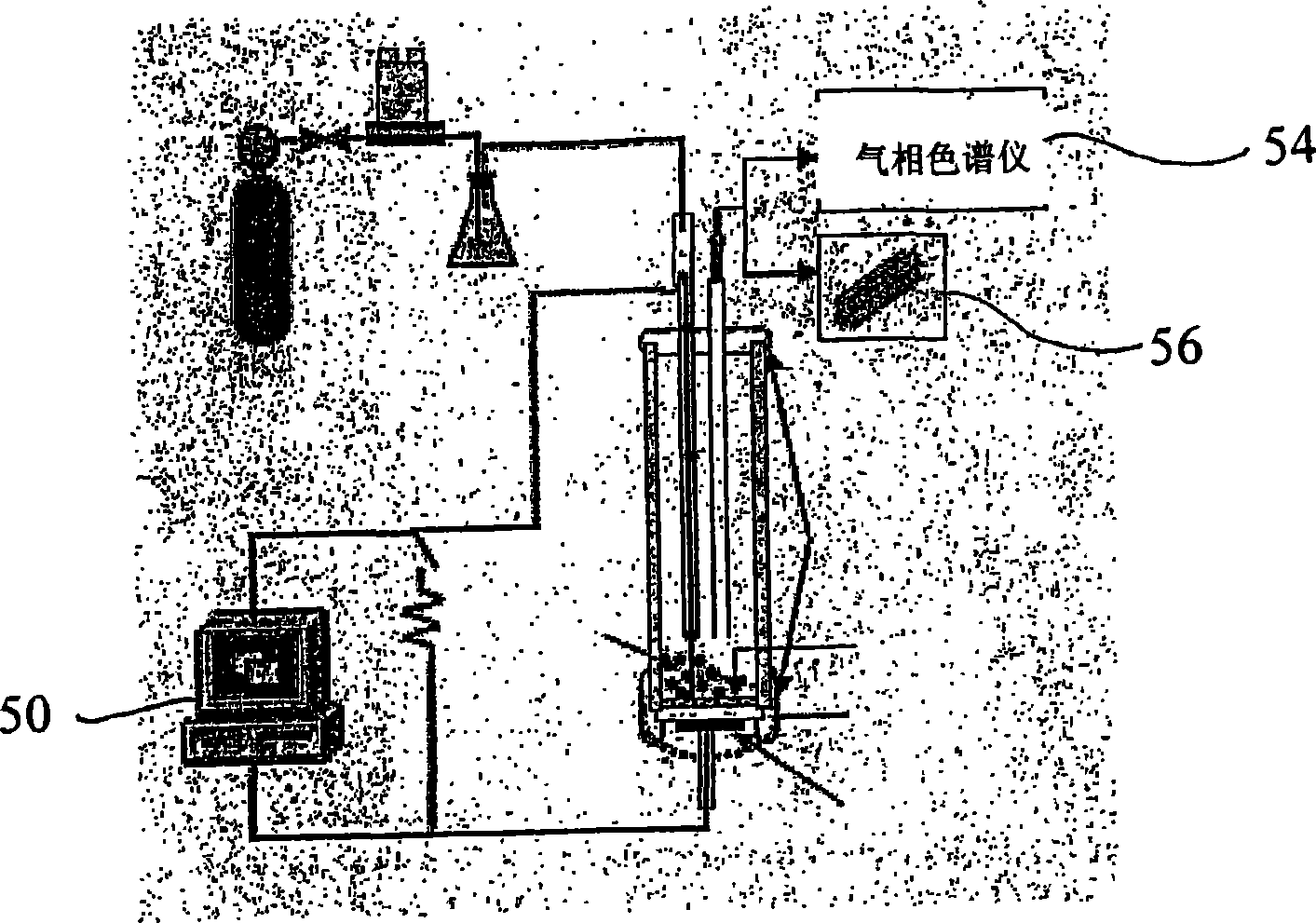
[0045] By having interface and Labview TM The software PC 50 obtains current and voltage output data from the fuel cell 10 . Gaseous exhaust products were analyzed by an SRI 8610C gas chromatograph54 and a Pfeiffer QMS200 mass spectrometer56. Analysis of gaseous products such as CO and CO2 allows determination of fuel conversion efficiency and by-product formation.
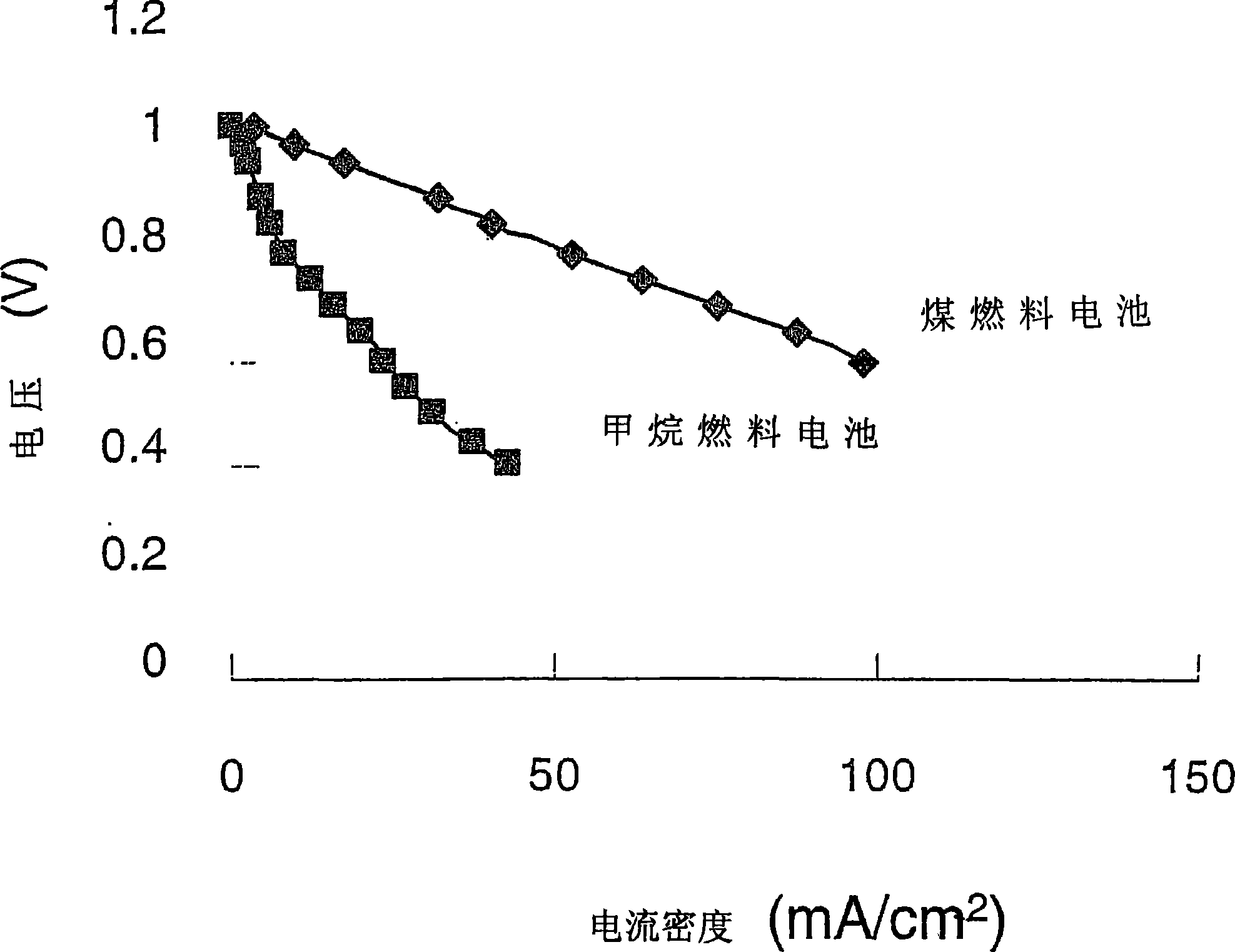
[0046] refer again figure 2 , shows the use of pure CH 4 Performance (I-V curves) of fuel cell 10 fueled with Ohio No. 5 coal (Table 1). Using coal as solid organic fuel, the coal is loaded into the tubular box 24 through the inlet 25 and ...
Embodiment 2
[0075] A YSZ disk (YSZ disk) with a thickness of 1 mm was purchased from Tosoh Inc. and used as the solid oxide electrolyte 18 . Ni(NO 3 ) 2 and NH 4 ReO 4 Impregnation prepares the materials that form the anode 14 and electrochemical oxidation catalyst assembly. The nominal weight percents of Ni and Re on the anode material are 5wt% (wt% means weight percent) and 2wt%, respectively. The anode / electrochemical oxidation catalyst material was pasted on the surface of a YSZ disc (1000 microns) with glycerin and calcined at 1000°C for 4 hours. This procedural process is repeated twice for making the thin layer forming the anode 14 .
[0054] A first electrode material was prepared by mixing lanthanum strontium manganese oxide (LSM-20, NexTech Materials) with YSZ powder in a 1:1 ratio. The cathode 12 was formed by pasting this first electrode material on a YSZ disc using glycerin, and calcined at 950° C. for 4 hours.
Solid Oxide Fuel Cell Assembly
[0055] Wi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com