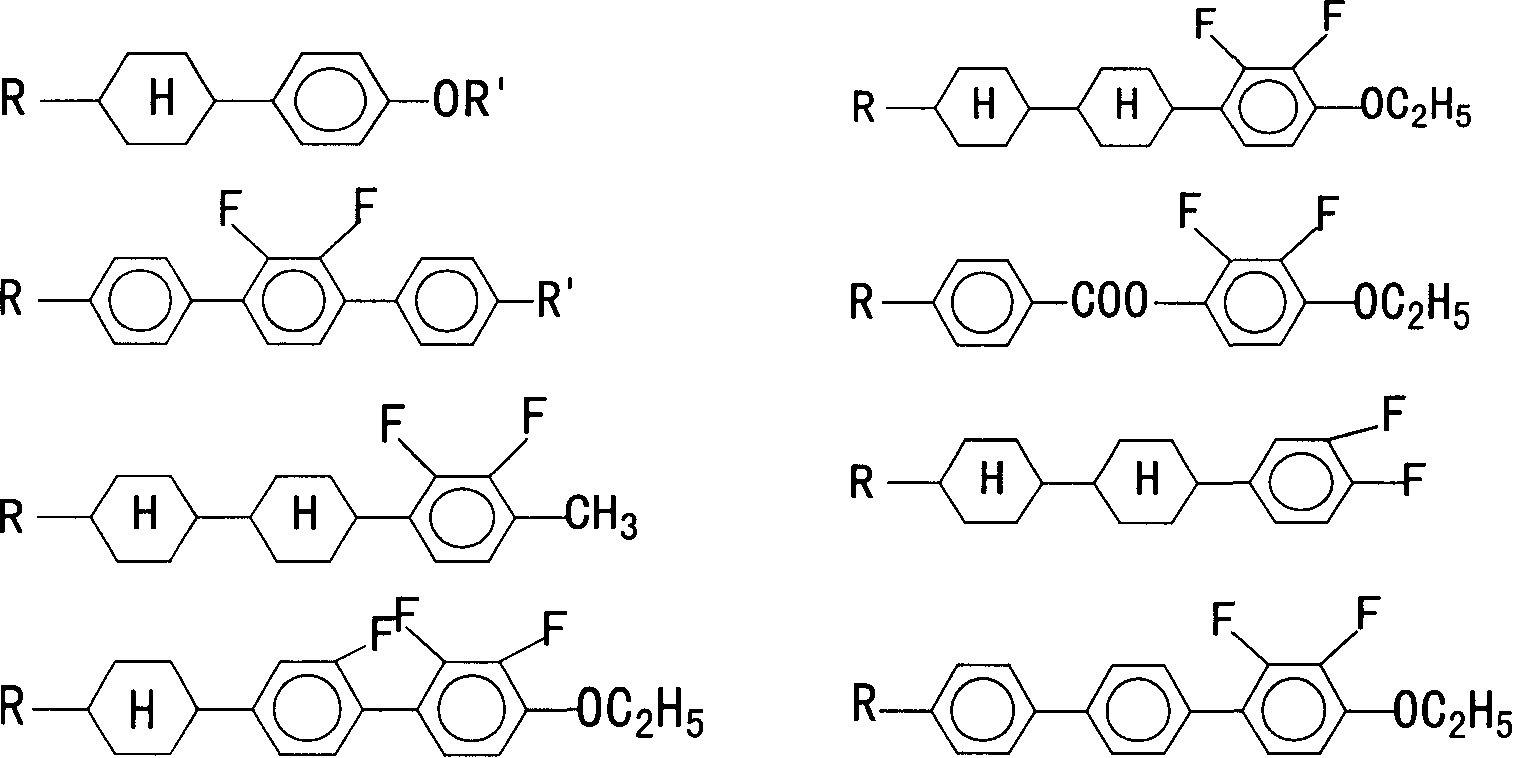
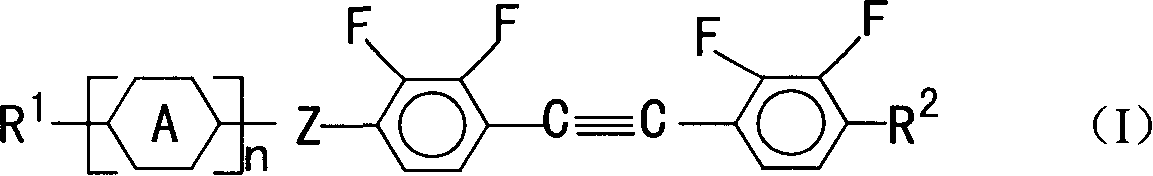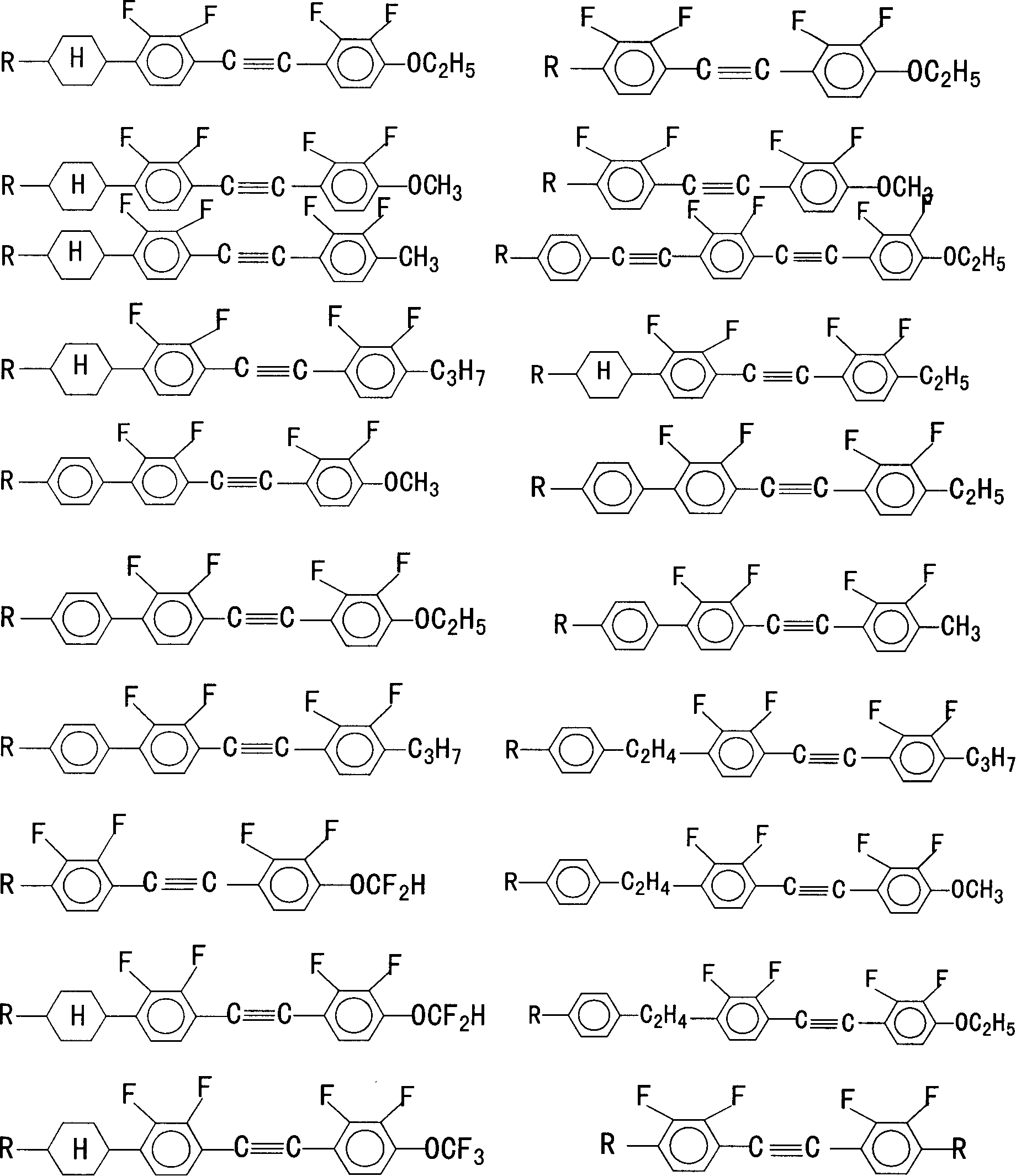2,3,2,'3' tetrafluoro diphenyl acetylene derivative, its composition, preparation method and use
A technology of tetrafluorotoluene and its derivatives, which is applied in 2 fields and can solve problems such as insufficient effectiveness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0094] Preparation of 4-propyl-4`-ethoxy-2,3,2`,3`-tetrafluorobenzil.
[0095]
[0096] Step 1-1: Synthesis of 4-ethoxy-2,3-difluoroiodobenzene: Add 52g (0.37mol) 2,3-difluorophenetole, 250ml dry tetrafluorofuran into a three-necked flask, and lower the temperature under nitrogen protection At -70°C, start to add 0.45mol of n-butyllithium dropwise for about half an hour. After the addition is complete, keep it at -70°C for 1.5 hours. Add dropwise for 1 hour, after dropping, keep for 1 hour; slowly add 10% NaHSO 3 Solution until discolored, separated, the organic layer was washed twice with water, anhydrous MgSO 4 Dry, filter off the desiccant, evaporate to dryness to obtain 88g, gas chromatography purity: 95%, yield: 81%.
[0097]Step 2-1: Synthesis of 4-propyl-2,3-difluoroiodobenzene: Add 117g (0.75mol) propyldifluorobenzene and 600ml dry THF into a three-necked flask, and cool down to -70°C under nitrogen protection Start to add 0.9 mol of n-butyllithium dropwise, cont...
Embodiment 2
[0105] Preparation of trans-4-propylcyclohexyl-4`-ethoxy-2,3,2`,3`-tetrafluorobenzil.
[0106]
[0107] Step 1-1: Synthesis of propylcyclohexene-2,3-difluorobenzene: Add 114g (1mol) of o-difluorobenzene and 300ml of dry THF into a three-necked flask, install and stir, cool down to -70°C under nitrogen protection, and start Add 1.1mol of n-butyllithium dropwise, control the temperature at -70°C, add dropwise for about 1 hour, after dropping, keep for 1 hour, then add 126g (0.9mol) of acetone dropwise at -70°C Add dropwise for about 1 hour, and keep for 4-5 hours after the drop is completed. Pour into about 1.5L of water for hydrolysis, extract with 500ml×3 toluene, combine organic phases, wash with water until neutral, divide into clean water, add another three-neck flask, add 5g of p-toluenesulfonic acid, heat up while stirring, and distill THF , when the internal temperature reaches 108°C, stop the distillation, change to a reflux condenser, and reflux for 5-6 hours. Aft...
Embodiment 3
[0120] Preparation of trans 4-pentylcyclohexyl-4`-ethoxy-2,3,2`,3`-tetrafluorobenzil
[0121]
[0122] With reference to the synthetic method in Example 2, the difference is that the acetone in step 1-1 is replaced with pentanecyclone, reacted with 2,3-difluorophenyllithium reagent, and undergoes dehydration, hydrogenation, transposition, Iodination gives 4-pentylcyclohexyl-2,3-difluoroiodobenzene, which is then coupled with 4-ethoxy-2,3-difluorophenylacetylene, and its total yield is 38%. The experimental results are as follows:
[0123] (1) Assay by gas chromatography: >99.7%.
[0124] (2) Compound phase structure: C: 84.5°C N: 208.7°C I. I
[0125] molecular formula
Characteristic ions (M / Z + ) and abundance (%)
C 27 h 30 f 4 o
446(M+,100),390(14.80),305(5.78),292(42.71),279(20.15),266(9.78),
[0126] Elemental analysis:
[0127] Theoretical calculated values: C: 72.63%, H: 6.77%.
[0128] Experimental values: C: 72.61%, H: ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com