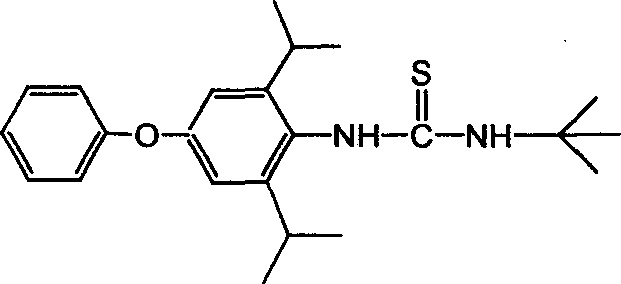
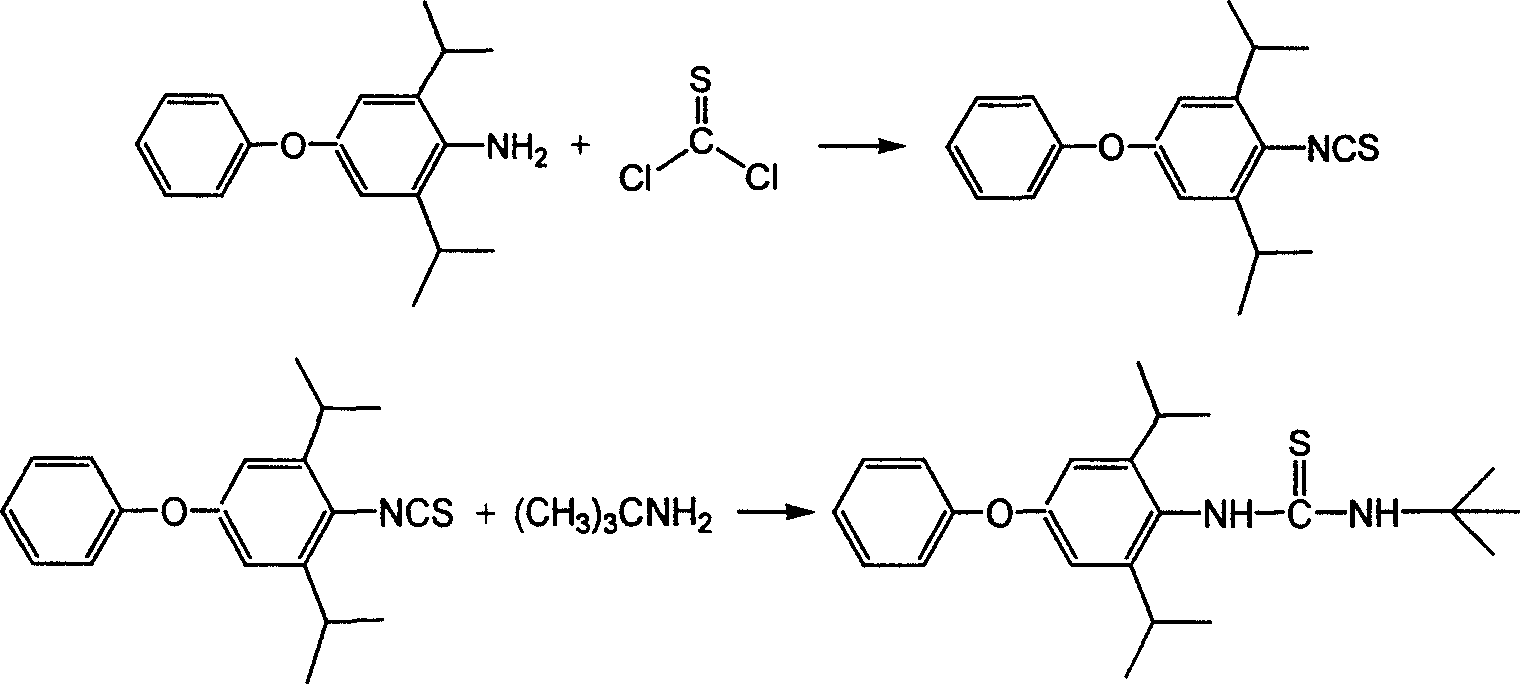
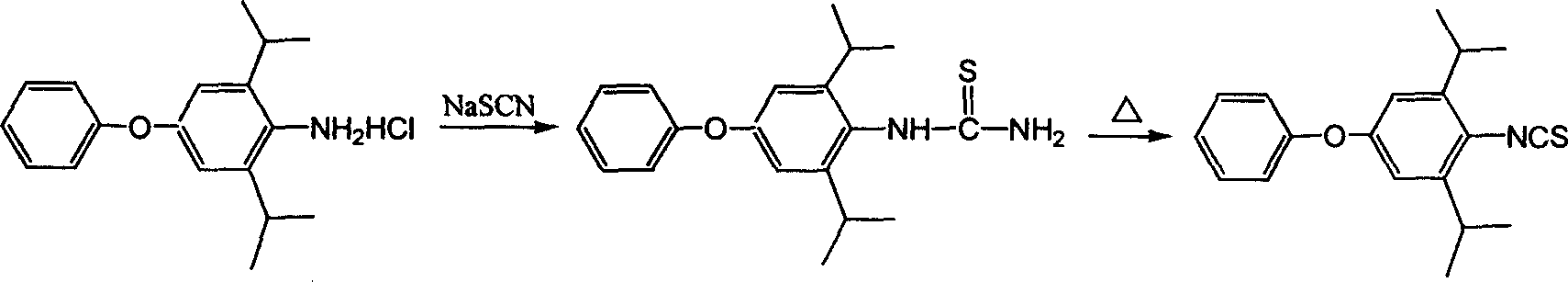
Method for preparing 1-tert butyl-3-(2,6-diisopropyl-4-phenyl cxypheny) thiourea
A technology of phenoxyphenyl and diisopropylaniline, which is applied in the field of preparation of thiourea organic compounds, can solve the problems of many reaction by-products, high raw material prices, high toxicity, etc., and achieves high total yield and low cost The effect of fewer energy and reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] (1) Add 4-phenoxy-2,6-diisopropylaniline 4.2g (content 91%, 0.014mol), carbon disulfide 4g (content 99%, 0.052mol), triethylamine 5.3g to the reaction flask (content 99%, 0.052 mol) and toluene 10ml, stirred at 20~25 ℃ for about 14 hours, when the content of 4-phenoxy-2,6-diisopropylaniline in the raw material monitored by HPLC was less than 1%, the reaction ended. A yellow solid precipitated out in the reaction flask and was filtered. The filtrate was reserved for the next application; the filter cake was dried to obtain 6.2 g of solid as triethylamine dithio(4-phenoxy-2,6-diisopropylanilino)formate.
[0030] (2) Put the triethylamine dithio(4-phenoxy-2,6-diisopropylphenylamino)formate solid obtained in the previous step into the reaction flask, add 10ml tert-butylamine and 10ml triethylamine at 20~ Stir at 25°C for about 24 hours, and when the content of triethylamine dithio(4-phenoxy-2,6-diisopropylanilino)formate in the raw material monitored by HPLC is less than 1...
Embodiment 2
[0032] (1) Add 4-phenoxy-2,6-diisopropylaniline 21.0g (content 93%, 0.07mol), carbon disulfide 21.6g (content 99%, 0.28mol) and toluene 100ml in 250ml autoclave, After ammonia replacement, at 20-25°C, 8kg / cm 2 The reaction was continued under pressure with ammonia stirring for about 24 hours. When the content of 4-phenoxy-2,6-diisopropylaniline hydrochloride in the raw material monitored by HPLC was less than 1%, the reaction ended. A yellow ammonium dithio(4-phenoxy-2,6-diisopropylanilino)formate solid precipitated out of the reaction mixture.
[0033] (2) Put the yellow reaction mixture obtained in the previous step into a reaction flask, add 10ml of tert-butylamine and stir at 20-25°C for about 24 hours. The raw material dithio(4-phenoxy-2,6-diisopropyl When the ammonium salt content of anilino)formate is less than 1%, the reaction ends. Excess tert-butylamine and solvent toluene were removed by distillation under reduced pressure, which will be used in the next step; 40m...
Embodiment 3
[0035] (1) Add 23.7g (content 93%, 0.072mol) of 4-phenoxy-2,6-diisopropylaniline hydrochloride to the reaction flask, then add 20ml ammonia (25%) and 25ml water, Add 21.6 g of carbon disulfide (content 99%, 0.28 mol) dropwise under stirring, stir at 20-25°C for about 24 hours, and monitor the content of the raw material 4-phenoxy-2,6-diisopropylaniline hydrochloride by HPLC to be less than 1 %, the reaction ends. A yellow solid precipitated out in the reaction flask and was filtered. The filtrate was reserved for the next application; the filter cake was dried to obtain 25.9 g of solid as dithio(4-phenoxy-2,6-diisopropylanilino)ammonium formate.
[0036](2) Put the solid obtained in the previous step into a reaction flask, add 30 ml of tert-butylamine and stir at 20-25°C for about 24 hours. The raw material dithio(4-phenoxy-2,6-diisopropylbenzene When the amino) ammonium formate content is less than 1%, the reaction ends. Distill under reduced pressure to remove excess tert...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com