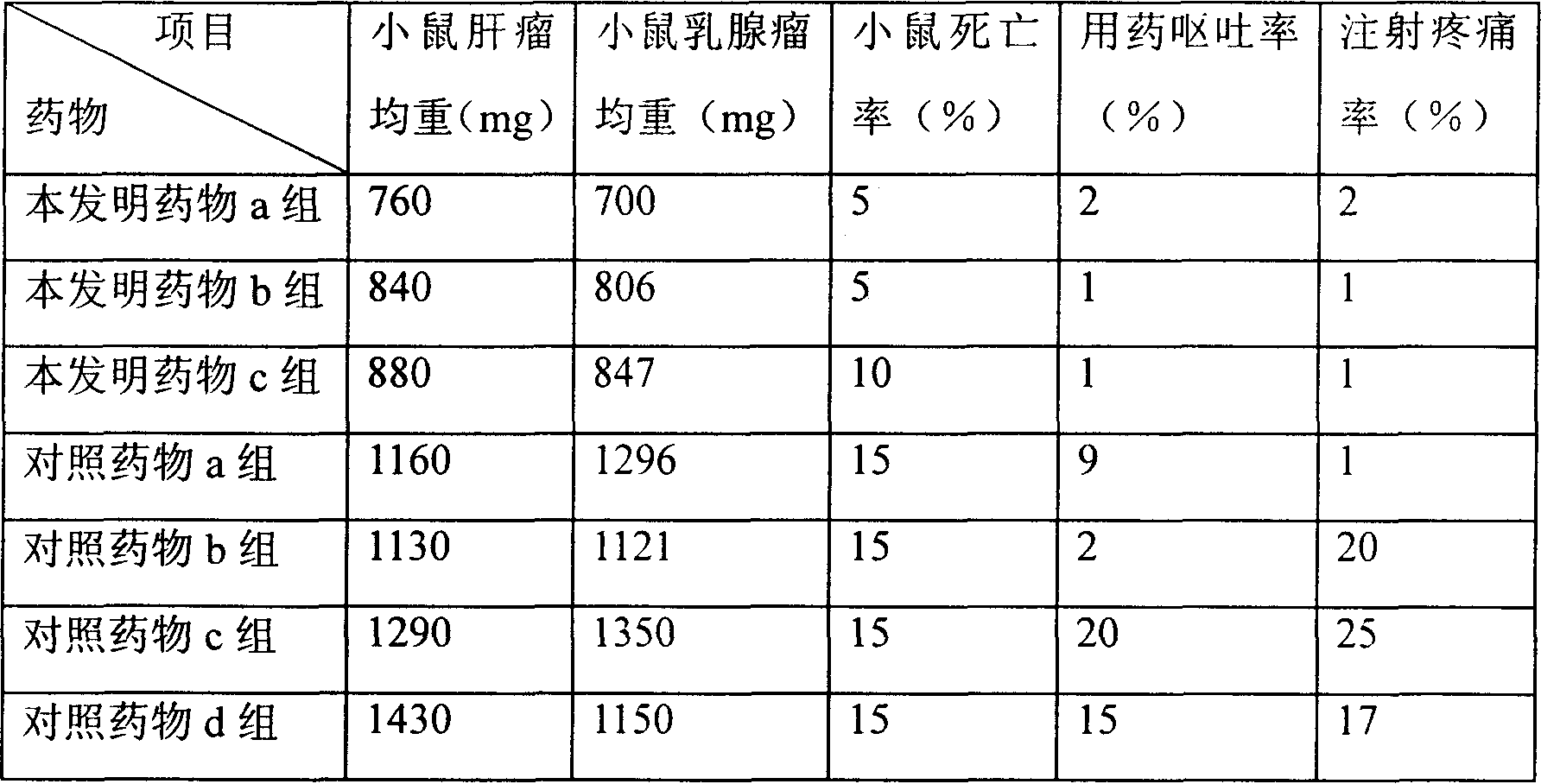
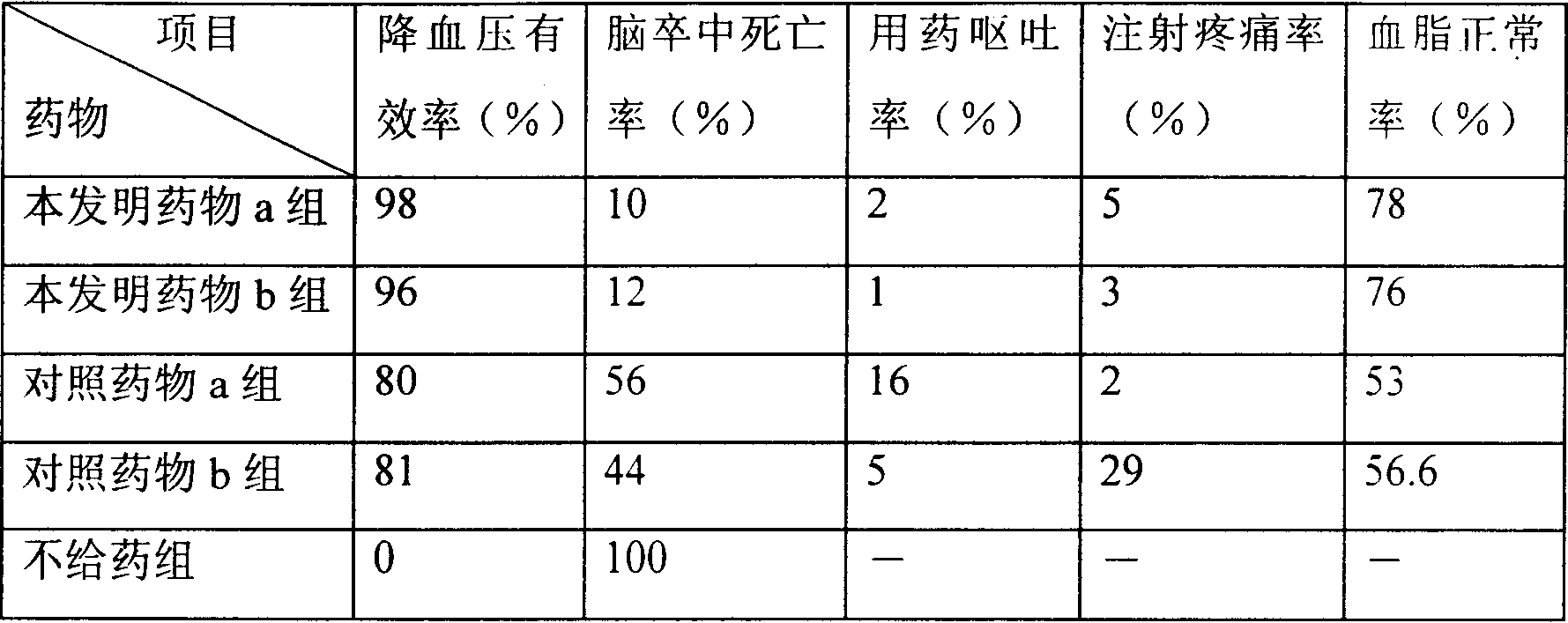
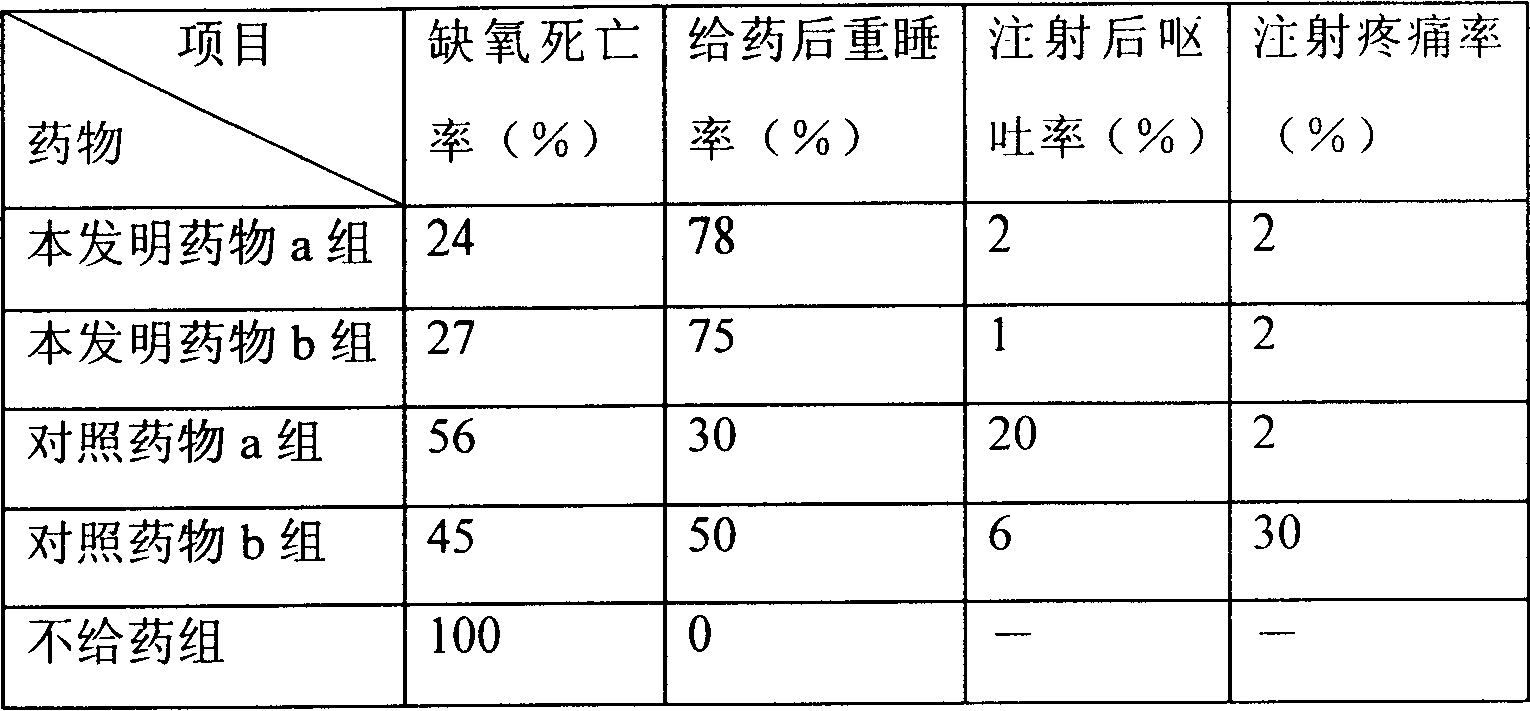
Composition of liposome, and preparation method
A technology of composition and plastid, which is applied in the field of liposome composition and its preparation, can solve the problems of serious adverse drug reactions, etc., and achieve the effects of reducing drug dosage, reducing cost and prolonging metabolic time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] The formula that present embodiment adopts is as follows:
[0035] Aspirin 20000g;
[0036] Alprostadil 1g;
[0037] Antioxidant 1000g;
[0038] The mixture of 2-hydroxypropyl-β-cyclodextrin and γ-cyclodextrin 2000g;
[0039] Hydrogenated soybean lecithin and egg yolk lecithin 1: 2 weight ratio mixture 6000g;
[0040] Soy sterol 3000g;
[0041] Polyethylene glycol-2000 2000g;
[0042] Vitamin C and glycine weight ratio 3: 1 mixture 4000g.
[0043] The antioxidant is a mixture of vitamin E and gamma-linolenic acid or dihomo-gamma-linolenic acid in any weight ratio. Wherein the aspirin may be aspirin lysine, aspirin arginine, and its weight is calculated by aspirin.
[0044] The preparation method steps are as follows:
[0045] (1) dissolving the phospholipid mixture, soy sterol, and antioxidant of formula quantity into solution with dehydrated alcohol or ether;
[0046] (2) Put the solution prepared in step (1) in a rotary evaporator, and evaporate to dryness at...
Embodiment 2
[0088] The formula adopted in this embodiment:
[0089] Aspirin 3500mg;
[0090] Alprostadil 350mg;
[0091] Antioxidant 180g;
[0092] Mixture of 2-hydroxypropyl-β-cyclodextrin and γ-cyclodextrin 250g;
[0093] Hydrogenated soybean lecithin and egg yolk lecithin 1: 3 weight ratio mixture 900g;
[0094] Soy sterol 600g;
[0095] Polyethylene glycol-2000 420g;
[0096] Vitamin C and glycine weight ratio 3: 1 mixture 560g.
[0097] The antioxidant is a mixture of vitamin E and gamma-linolenic acid or dihomo-gamma-linolenic acid in any weight ratio. γ-linolenic acid is also known as vitamin F. Dihomo-γ-linolenic acid is also called arachitrienoic acid.
[0098] Wherein the aspirin may be aspirin lysine, aspirin arginine, and its weight is calculated by aspirin.
[0099] The present invention also provides the preparation method of described aspirin and alprostadil liposome composition, and the steps are as follows:
[0100] (1) dissolving the phospholipid mixture, soy s...
Embodiment 3
[0144] The formula that present embodiment adopts is as follows:
[0145] Aspirin 20000g;
[0146] Alprostadil 5g;
[0147] Antioxidant 1000g;
[0148] The mixture of 2-hydroxypropyl-β-cyclodextrin and γ-cyclodextrin 3000g;
[0149] Hydrogenated soybean lecithin and egg yolk lecithin 1: 4 weight ratio mixture 6000g;
[0150] Soy sterol 8000g;
[0151] Polyethylene glycol-2000 2000g;
[0152] Vitamin C and glycine weight ratio 3: 1 mixture 6000g.
[0153] The antioxidant is a mixture of vitamin E and gamma-linolenic acid or dihomo-gamma-linolenic acid in any weight ratio. Wherein the aspirin may be aspirin lysine, aspirin arginine, and its weight is calculated by aspirin.
[0154] The preparation method steps are as follows:
[0155] (1) dissolving the phospholipid mixture, soy sterol, and antioxidant of formula quantity into solution with dehydrated alcohol or ether;
[0156] (2) Put the solution prepared in step (1) in a rotary evaporator, and evaporate to dryness at...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com