Matrix for sustained, invariant and independant release of active compounds
A matrix and composition technology, applied in the direction of organic active ingredients, digestive system, drug combination, etc., can solve the problems of limited range of analgesic dosage, different release distribution, and inability to ensure release distribution, etc., to reduce side effects and simplify the preparation method Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
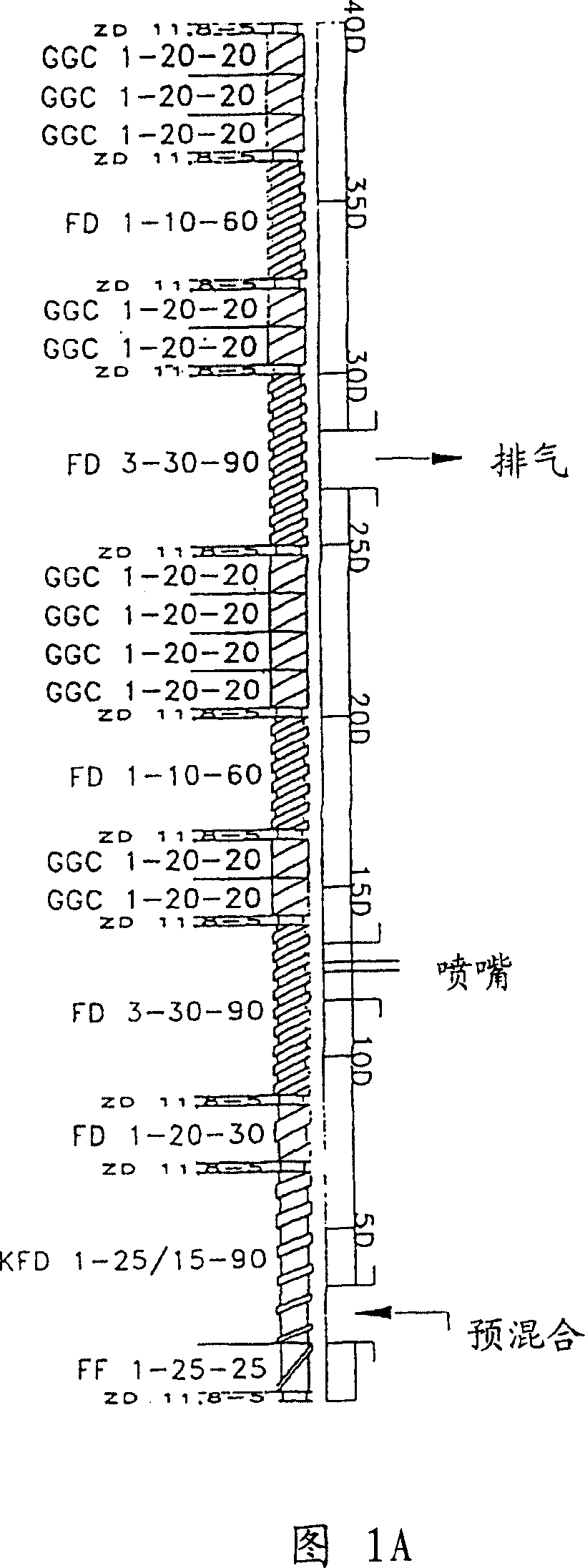
[0161] Example 1 - By spray granulation to produce different hydroxyl groups in non-swellable diffusion matrices Codone / naloxone dosage tablet
[0162] The following component amounts can be used to make the oxycodone / naloxone tablets of the present invention.
[0163] Preparation (name)
[0164] The Surelease(R) E-7-7050 polymer blend used had the following composition.
[0165] Surelease®
[0166] For the preparation of tablets, Oxycodone HCl, Naloxone HCl, Povidone 30 and Lactose FlowLac100 were mixed in a tumbler mixer (Bohle) followed by spray granulation with Surelease® in a fluid tank granulator (GPCG3) change. This material was screened through a Comill 1.4 mm sieve. An additional granulation step was performed with molten fatty alcohol in a high-cutting mixture (Collette). All tablet cores made by this procedure had a weight of 123 mg on a dry matter basis.
Embodiment 2
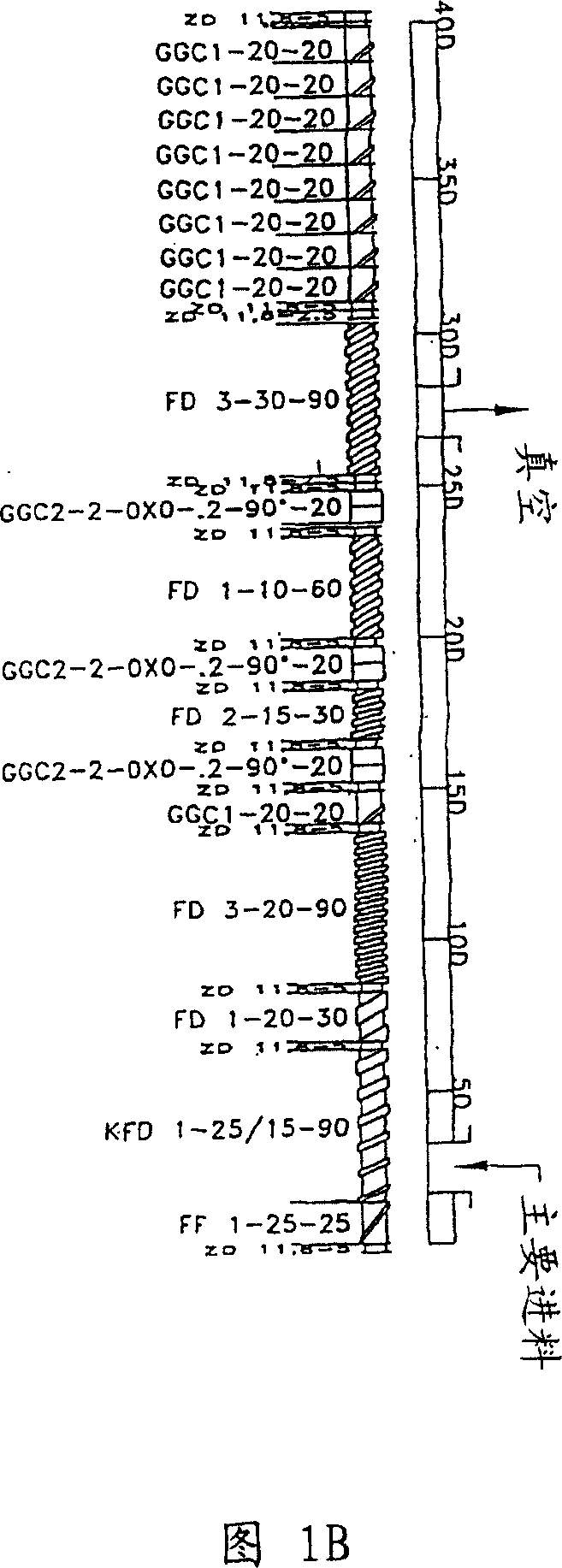
[0167] Embodiment 2-by extruding to make have oxycodone and nalox in non-swellable diffusion matrix ketone dose tablets
[0168] The following component amounts can be used to make the oxycodone / naloxone tablets of the present invention.
[0169] Preparation (name)
Oxy / Nal-Extr
Oxycodone HCl
20mg
Naloxone HCl
10mg
Kollidone 30
6 mg
Lactose Flow Lac100
49.25 mg
Ethyl cellulose 45cpi
10mg
24 mg
2.5 mg
1.25 mg
[0170] The above amounts of Oxycodone HCl, Naloxone HCl, Ethylcellulose 45 cpi, Kollidone 30, Stearyl Alcohol and Lactose Flow Lac100 were mixed in a drum mixer (Bohle). The mass was subsequently extruded with a counter-rotating twin-screw extruder of type Micro 18GGL (Leistritz AG, Nuernberg, Germany). The temperature of heating zone 1 was 25°C, heating zone 2 was 50°C, heating zone 3 to 5 was 60°C, heating zone 6 to 8 ...
Embodiment 3
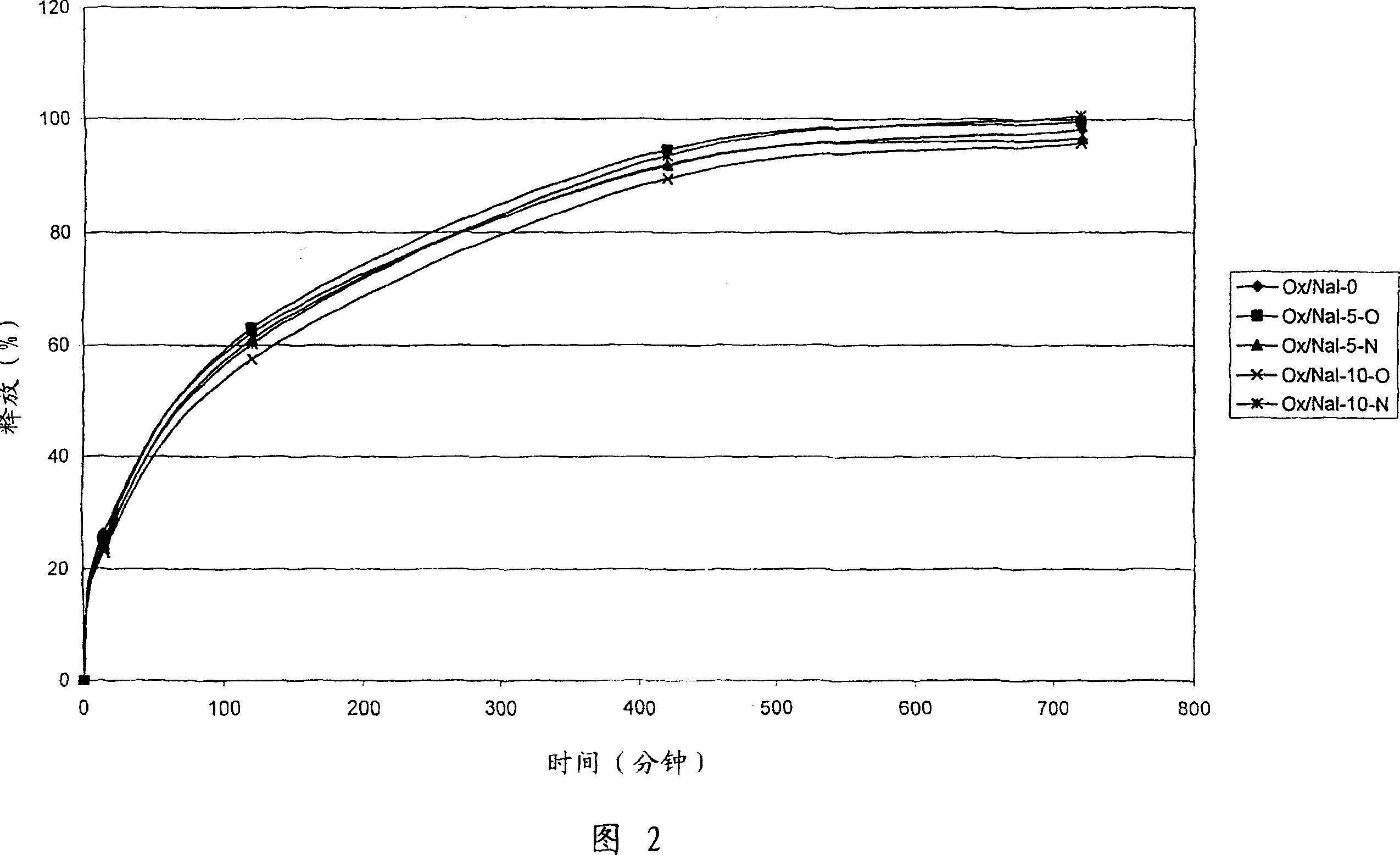
[0172] Example 3 - Release Profile of Oxycodone / Naloxone Tablets Taken from Example 1:
[0173] The release of the active compound was measured over a period of 12 hours using HPLC at pH 1.2 and applying the Basket method of USP. Test tablets Ox / Nal-0, Ox / Nal-5 or Ox / Nal-10.
[0174] From Figure 2 and the values listed in the table, it follows that in the case of non-swellable expanded matrices based on Surelease(R), the release rate of different amounts of oxycodone remains the same regardless of the amount of naloxone. Correspondingly, a constant release profile of naloxone was observed at different oxycodone amounts.
[0175] time
[0176] Release values refer to oxycodone or naloxone (row 2) and are expressed as a percentage. The average release of naloxone at 420 minutes was 92.7%. The maximum error value is 1% at 420 minutes. Oxy and Nal stand for oxycodone and naloxone and denote the active compounds to be measured.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com