Methods and agents for inhibiting dynamin-dependent endocytosis
A dynamin and cell-inhibiting technology, applied in drug combinations, nitrile/isonitrile active ingredients, organic chemistry, etc., can solve problems such as weak specificity and limited effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
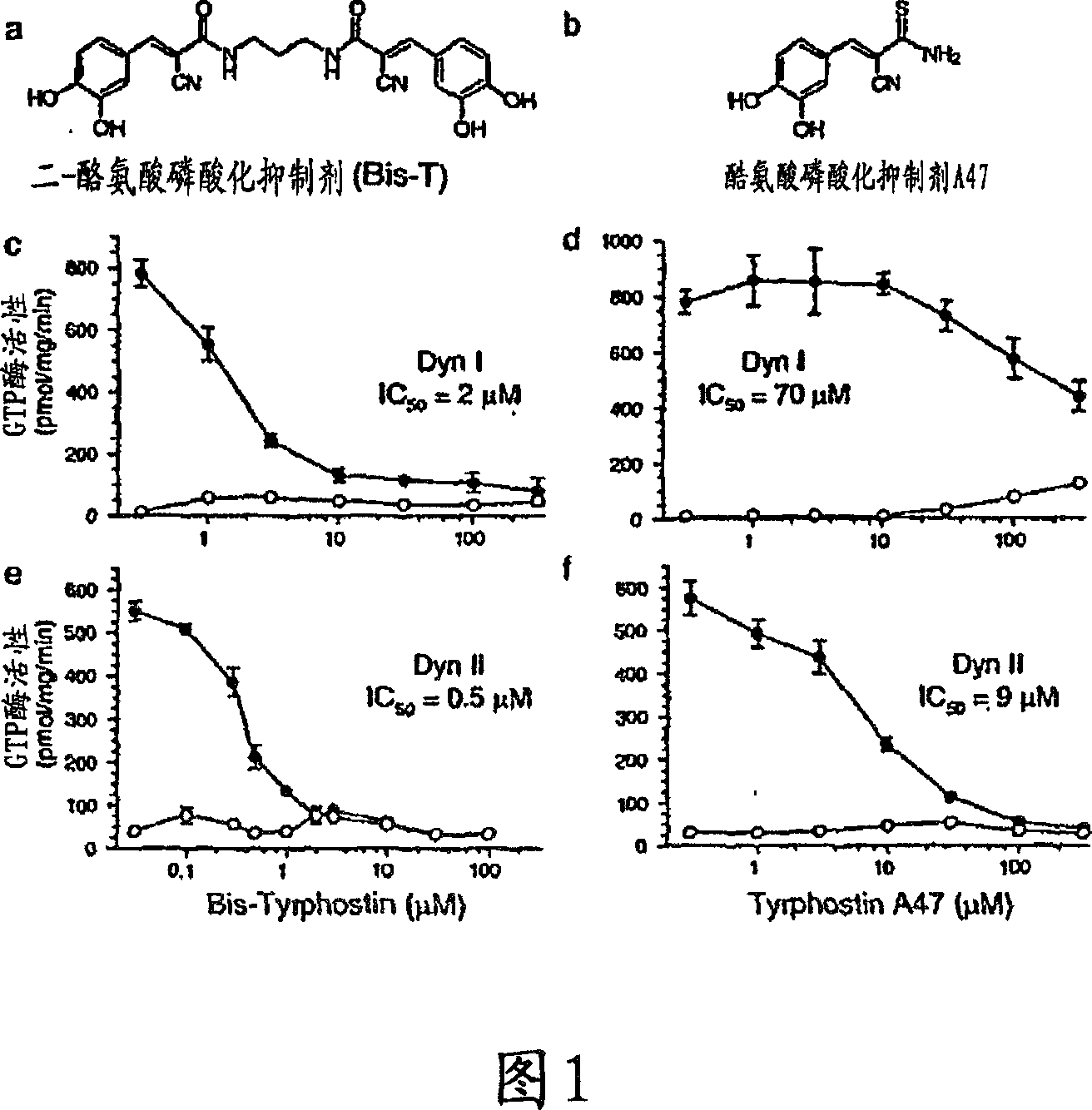
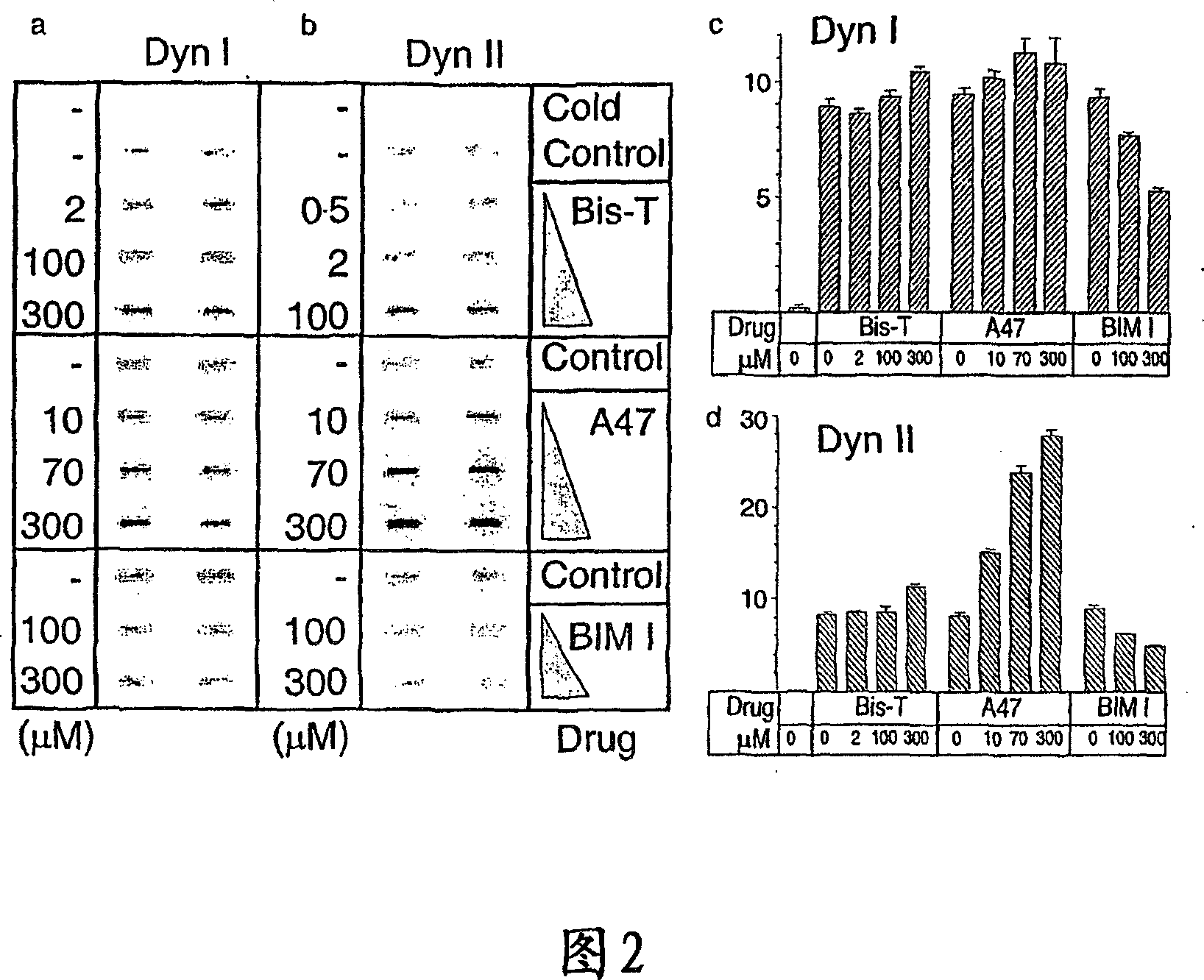
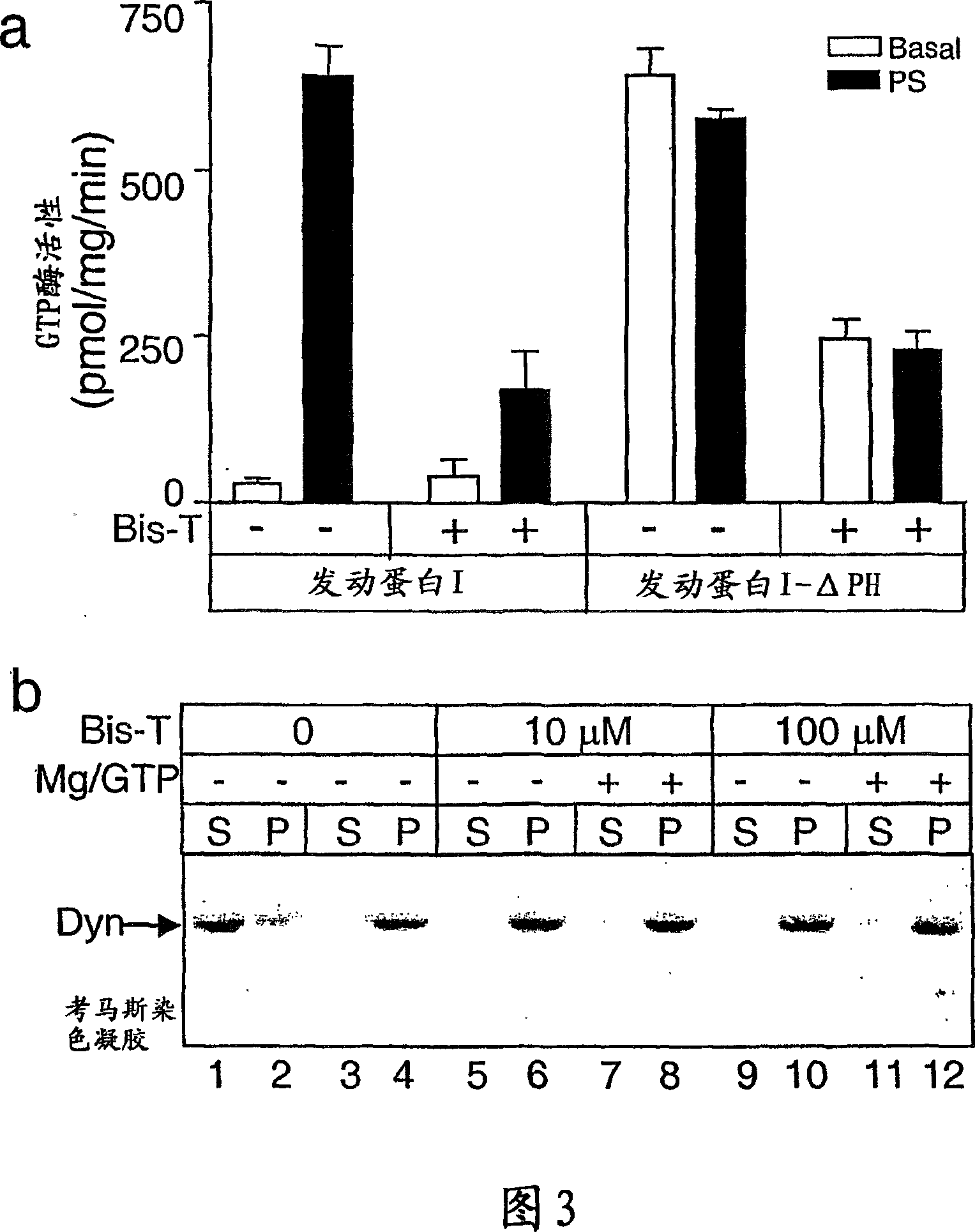
[0159] Example 1: Identification of Inhibition of Tyrosine Phosphorylation Inhibitors of Dynamin GTPase Activity
[0160] 1.1 Materials and methods
[0161] Phosphatidylserine, 1,2-glycerol dioleate, calmodulin, ATP, GTP, leupeptidase, phenylmethylsulfonyl fluoride, Tween 80, bis(sulfosuccinimidyl)octyl Basinate (BS3) and glutathione sepharose were obtained from Sigma. Papain and antipain dihydrochloride were obtained from Boehringer Mannheim (Federal Republic of Germany). Gel electrophoresis reagents and apparatus were from Bio-Rad. (3000 Ci / mmol) and (25 μCi / mmol) was obtained from Amersham plc, UK. Protein molecular weight markers and chromatography resins were from Pharmacia. All other reagents were of analytical grade or better.
[0162] 1.1.1 Protein preparation
[0163] The plasmid of GST-Amph2-SH3 (Muscle Amph2) (Butler et al., 1997) was provided by Pieto DeCamilli, Yale, Conneticut, USA, placed in pGEX2T vector. The plasmid was grown in E. coli and the GST-A...
Embodiment 2
[0200] Example 2: Development of Tyrosine Phosphorylation Inhibitor Analogs
[0201]
[0202] Di-Tyrosine Phosphorylation Inhibitor (1) and Tyrosine Phosphorylation Inhibitor A47 (2)
[0203] 2.1 Development of analogues
[0204] The structures of bis-tyrosine phosphorylation inhibitor and tyrosine phosphorylation inhibitor A47 are shown above. The structural similarity of these 3,4-dihydroxybenzene compounds and the presence of cyanamide or thioamide structures suggest that these groups are important for dynamin inhibition. These features can solve the problem of library development and synthesis, and there are two libraries for determining the type and number of aromatic substituents that determine activity, the need for symmetrical synthesis (1vs2), and the location of bis-tyrosine phosphorylation inhibitors. The importance of the length of the central alkane spacer between the two amino moieties These libraries are referred to as library 1 (dimeric compounds) and li...
Embodiment 3
[0315] Example 3: Development of Prodrugs
[0316] Prodrugs of bis-tyrosine phosphorylation inhibitors and their analogs were developed to increase the permeability of cell membranes and thus increase the potency of drugs in cells. A suitable reaction to provide a prodrug of a dimerized tyrosine phosphorylation inhibitor compound is illustrated in Scheme 5. Bis-tyrosine phosphorylation inhibitors are examples of initiating agents. The dimer tyrosine phosphorylation inhibitor compound is stirred together with the appropriate anhydride or acid chloride (in molar excess) in pyridine / N,N-dimethylformamide (DMF) solution in the presence of an appropriate catalyst such as dimethyl Aminopyridine (DMAP). In some cases, the solution required reflux to complete the reaction. After the reaction is complete, the esterified product is purified by recrystallization or chromatography. Examples of prodrugs developed are shown in Tables 2 and 3.
[0317]
[0318] Scheme 5. Synthesis of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com