Purification for separating feed gas in stabilized isotope 13C by low-temperature CO rectifying method
A low-temperature rectification and purification process technology, applied in the field of chemical production process, can solve the problems of inconvenient operation, complex equipment, high requirements for equipment conditions, etc., and achieve the effect of high purification efficiency, simple device and convenient use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
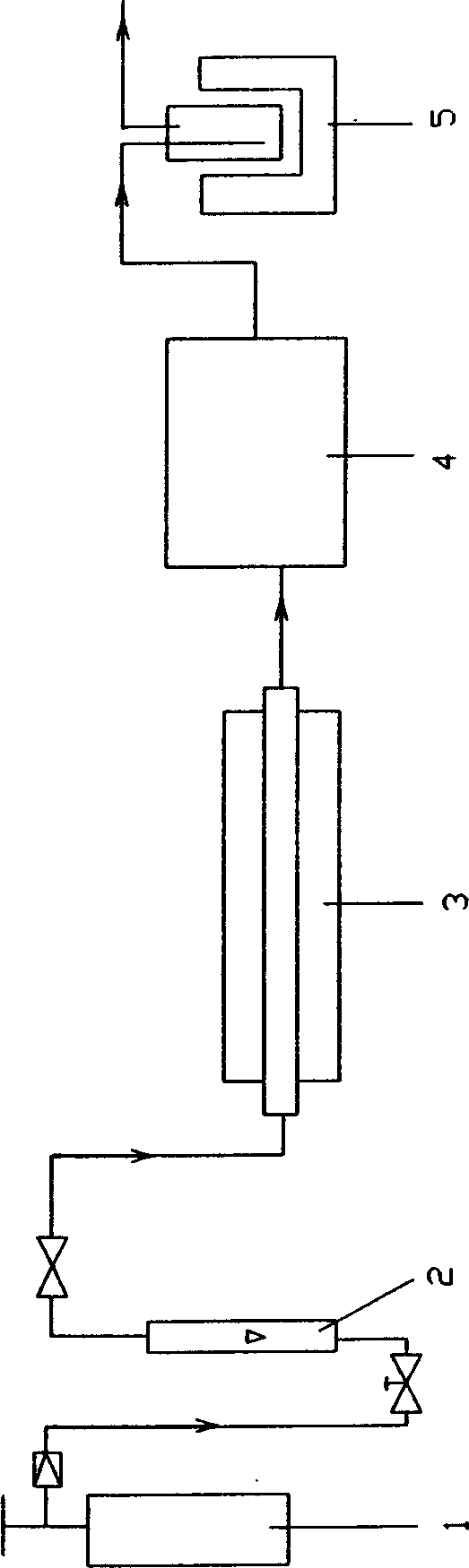
[0028] Cylinder CO feed gas contains O 2 50ppm, H 2 O 30ppm, CO 2 10ppm, N 2 500ppm, with a flow rate of 100L / h and a pressure of 0.15MPa, after passing through a molecular sieve normal temperature adsorber (the size of the adsorber is Φ50×1000, with 5A molecular sieve inside), the H 2 O≤1ppm, CO 2 ≤4ppm; then enter the chemical purification column (column size is Φ25×220, filled with Φ3mm sponge Ti particles), the purification column is heated to 700C to remove O in the raw gas 2 and further remove H 2 O and CO 2 , so that the O in the outlet gas 2 down to 0.1ppm, H 2O.CO 2 down to 0.1ppm; then enter the molecular sieve low-temperature adsorber (filled with 5A molecular sieve, particle size 4mm), deep purification at -190°C to obtain H 2 O.CO 2 , O 2 All≤0.01ppm; N 2 , Ar, and organic impurity gases are all ≤ 1ppm product gas.
Embodiment 2
[0030] O in industrial CO feed gas 2 ≤5%, H 2 O≤3%, CO 2 ≤1%, the rest is N 2 , Ar and organic impurity gas, with a flow rate of 140L / h and a pressure of 0.5MPa, after passing through a molecular sieve normal temperature adsorber (filled with 4A molecular sieve, particle size Φ4mm, adsorber is Φ500×5000), the H in the outlet gas 2 O≤4ppm, CO 2 ≤5ppm; then enter the chemical purification column (filled with Φ3mm manganese oxide particles, filling size Φ50×500), the purification column is heated to 200°C, O 2 The gas can be removed to 0.1ppm; then the gas enters the molecular sieve cryogenic adsorber (filled with 5A molecular sieve, particle size Φ4mm), and is deeply purified at -100°C to obtain H 2 O.CO 2 , O 2 All≤0.01ppm, while N 2 , Ar and organic impurities are all lower than 1ppm product gas.
Embodiment 3
[0032] O in industrial CO feed gas 2 ≤5%, H 2 O≤3%, CO 2 ≤1%, the rest is N 2 , Ar and organic impurity gas, with a flow rate of 120L / h and a pressure of 0.3MPa, after passing through the molecular sieve normal temperature adsorber 3 (filled with 13X molecular sieve, particle size Φ4mm, and the adsorber is Φ500×5000), the H in the outlet gas 2 O≤4ppm, CO 2 ≤5ppm; then enter the chemical purification column (filled with Φ3mm manganese oxide particles, filling size Φ50×500), the purification column is heated to 500°C, O 2 The gas can be removed to 0.1ppm; then the gas enters the molecular sieve cryogenic adsorber (filled with 5A molecular sieve, particle size Φ4mm), and is deeply purified at -150°C to obtain H 2 O.CO 2 , O 2 All≤0.01ppm, while N 2 , Ar and organic impurities are all lower than 1ppm product gas.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com