Compound, composition and method for preventing from neurodegeneration in injury of central nervous system
A compound and composition technology, applied in the field of neurological diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 5
[0057]
[0058] Reagent (a) N,N-methylformamide (DMF), triethylamine, room temperature, 4 hours.
[0059] Specific compounds of interest in formula (I) are as follows:
[0060] 5-Benzylaminosalicylic acid
[0061] 5-(4-nitrobenzyl)aminosalicylic acid (NBAS)
[0062] 5-(4-Chlorobenzyl)aminosalicylic acid (CBAS)
[0063] 5-(4-Trifluoromethylbenzyl)aminosalicylic acid (TBAS)
[0064] 5-(4-Fluorobenzyl)aminosalicylic acid (FBAS)
[0065] 5-(4-Methoxybenzyl)aminosalicylic acid (MBAS)
[0066] 5-(Pentafluorobenzyl)aminosalicylic acid (PBAS)
[0067] 5-(4-Nitrobenzyl)amino-2-hydroxybenzoic acid ethyl ester (NAHE)
[0068] 5-(4-Nitrobenzyl)-N-acetamido-2-hydroxybenzoic acid ethyl ester (NNAHE)
[0069] 5-(4-Nitrobenzyl)-N-acetamido-2-acetoxy-ethyl benzoate (NNAAE)
[0070] 5-(4-Nitrobenzoyl)aminosalicylic acid (NBAA)
[0071] 5-(4-Nitrobenzenesulfonyl)aminosalicylic acid (NBSAA)
[0072] 5-[2-(4-Nitrophenyl)-ethyl]aminosalicylic acid (NPAA)
[0073] 5-[3-(4-nitrophenyl)n...
experiment Embodiment 1
[0123] Experimental Example 1: Antioxidative Effect of 5-Aminosalicylic Acid
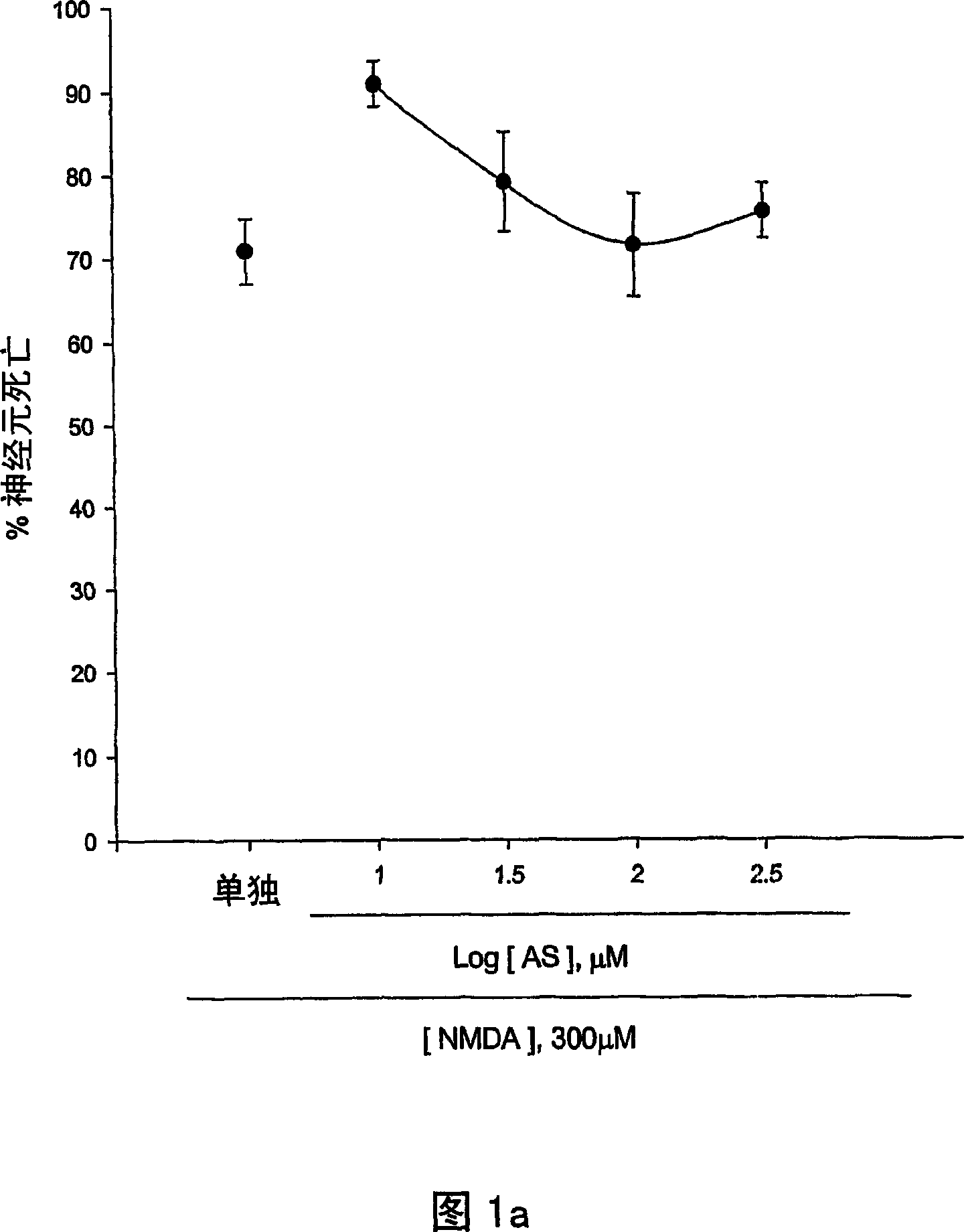
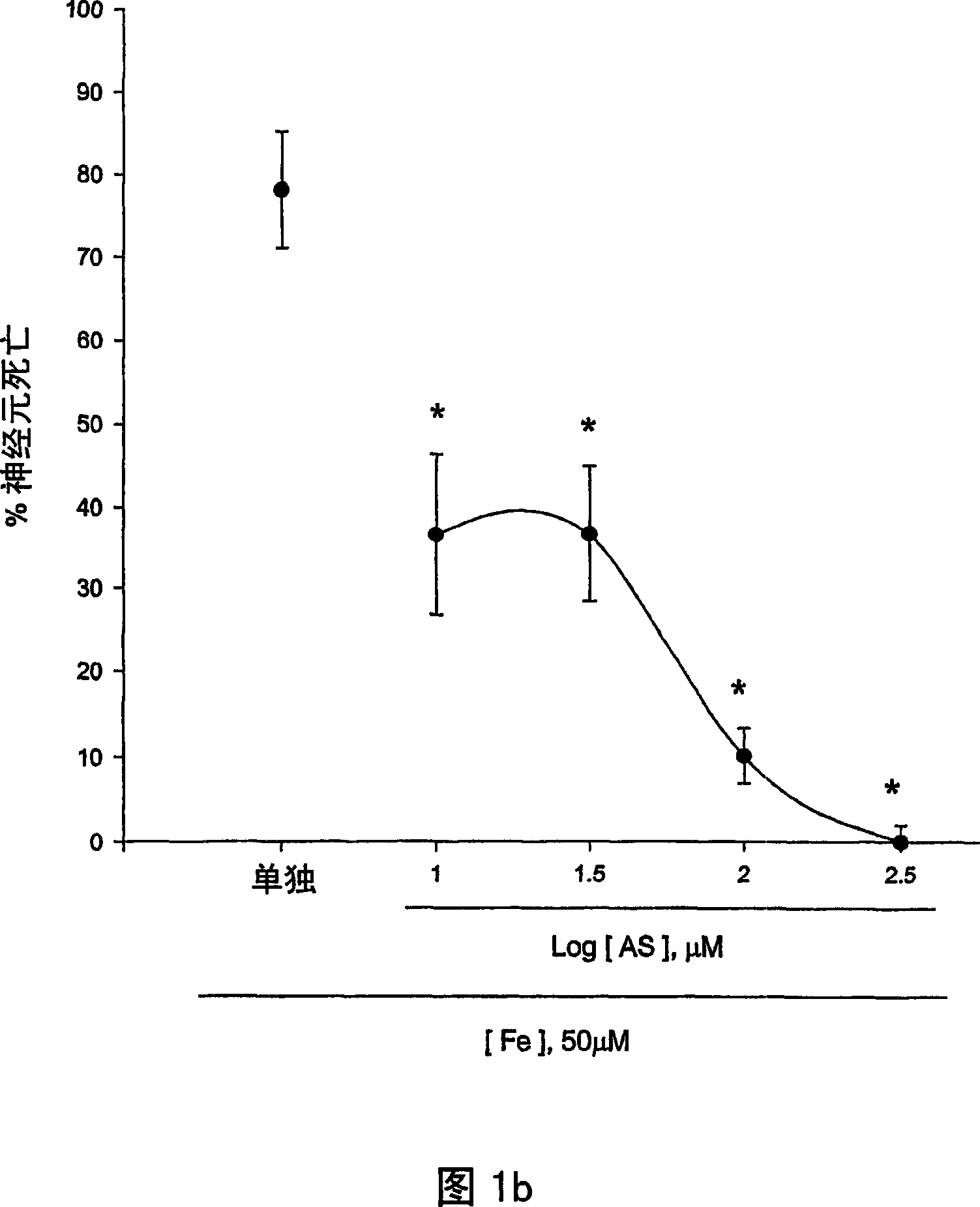
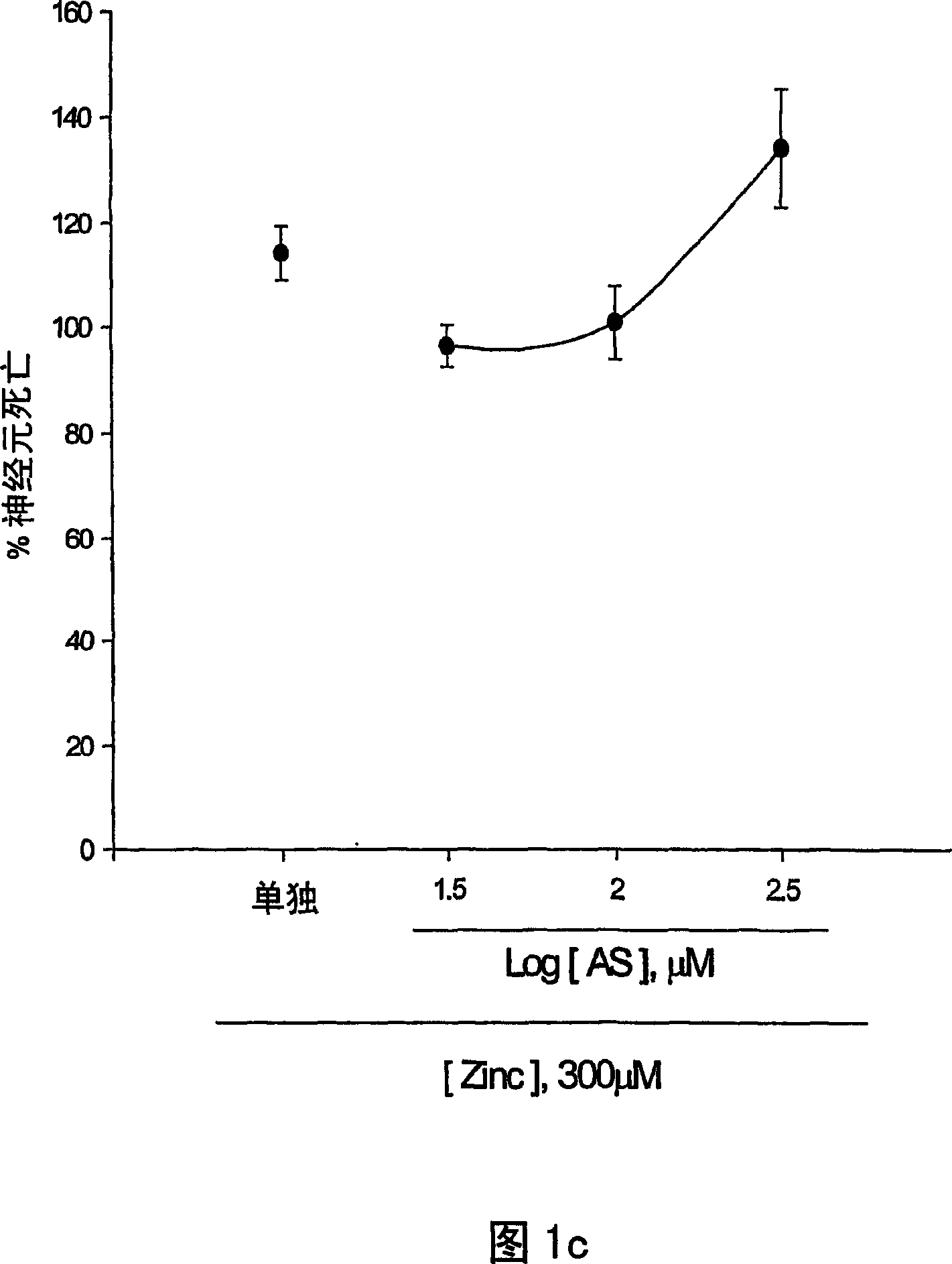
[0124] The neuroprotective effects of 5-aminosalicylic acid (AS) were tested in primary cortical cell cultures. Containing 10-300 μM AS did not reduce neuronal death induced by 10 min exposure to 300 μM NMDA over 24 hours (Fig. 1.a). Interestingly, adding 10-100 μM AS dose-dependently prevented free radical neurotoxicity induced by exposure to ferrous ions for 24 h (Fig. 1b). Continuous addition of AS during and after zinc ion treatment did not reduce neuronal death 24 hours after exposure to 300 micromolar zinc ions for 30 min. The neuroprotective effect of AS against free radical neurotoxicity is attributed to the direct antioxidant properties of the compound AS reducing the level of DPPH, a stable free radical. Compared to trolox, a membrane permeable form of vitamin E, AS is a weak oxidizing agent.
[0125] Reactant
Concentration of Trolox or AS (micromolar)
0
3...
experiment Embodiment 2
[0128] Experimental Example 2: Neuroprotective Effects of 5-Benzylaminosalicylic Acid and Its Derivatives
[0129] Neuroprotective effects of 1.5-benzylaminosalicylic acid
[0130] BAS was synthesized and tested for its ability to resist nerve damage induced in cortical cell cultures. Simultaneous addition of 300 micromolar 5-benzylaminosalicylic acid (BAS) was able to reduce NMDA-induced neuronal death by approximately 50% (Fig. 2a). In the presence of 1 μM BAS, there was a considerable decrease in neuronal death induced by exposure to 50 μM ferrous ions (Fig. 2b) or 10 mmol BSO (Fig. 2c), adding 3 μM molar BAs, neuronal death was essentially completely blocked. Addition of 30-300 micromolar BAS dose-dependently reduced neuronal death induced by exposure to 300 micromolar zinc ions for 30 minutes over 24 hours. Like AS or trolox, BAS is a direct antioxidant (Table 2). The antioxidant properties of BAS were observed at doses as low as 1 micromolar.
[0131] [BAS]...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com