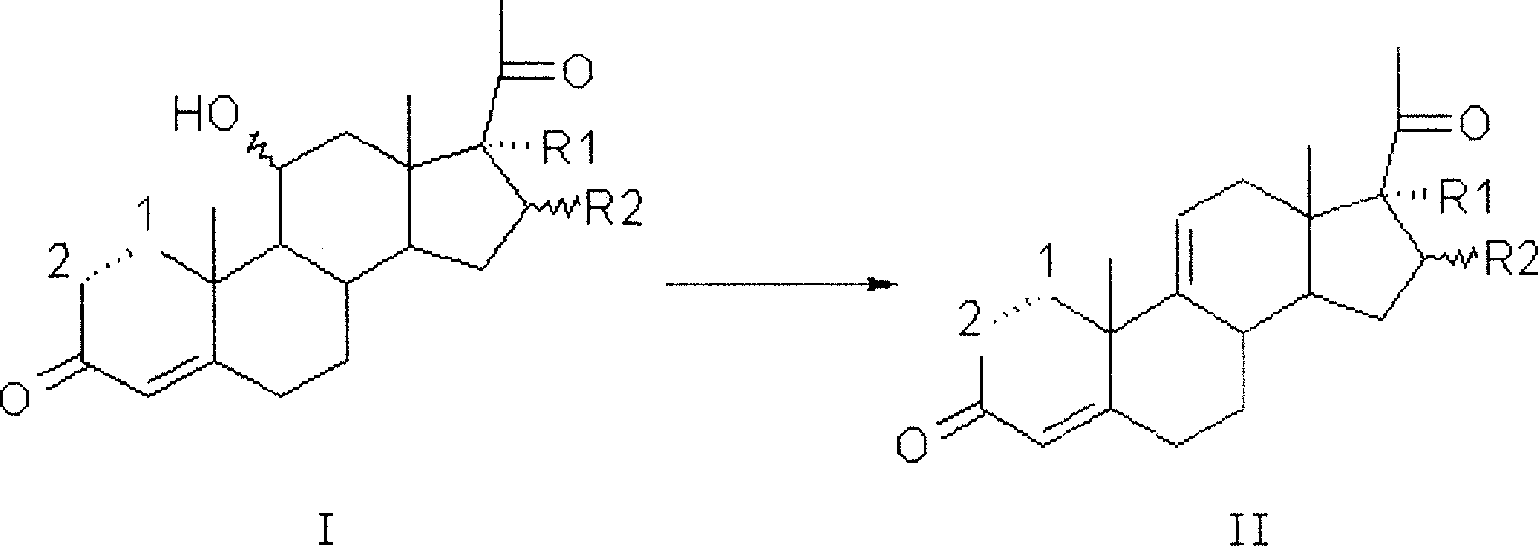
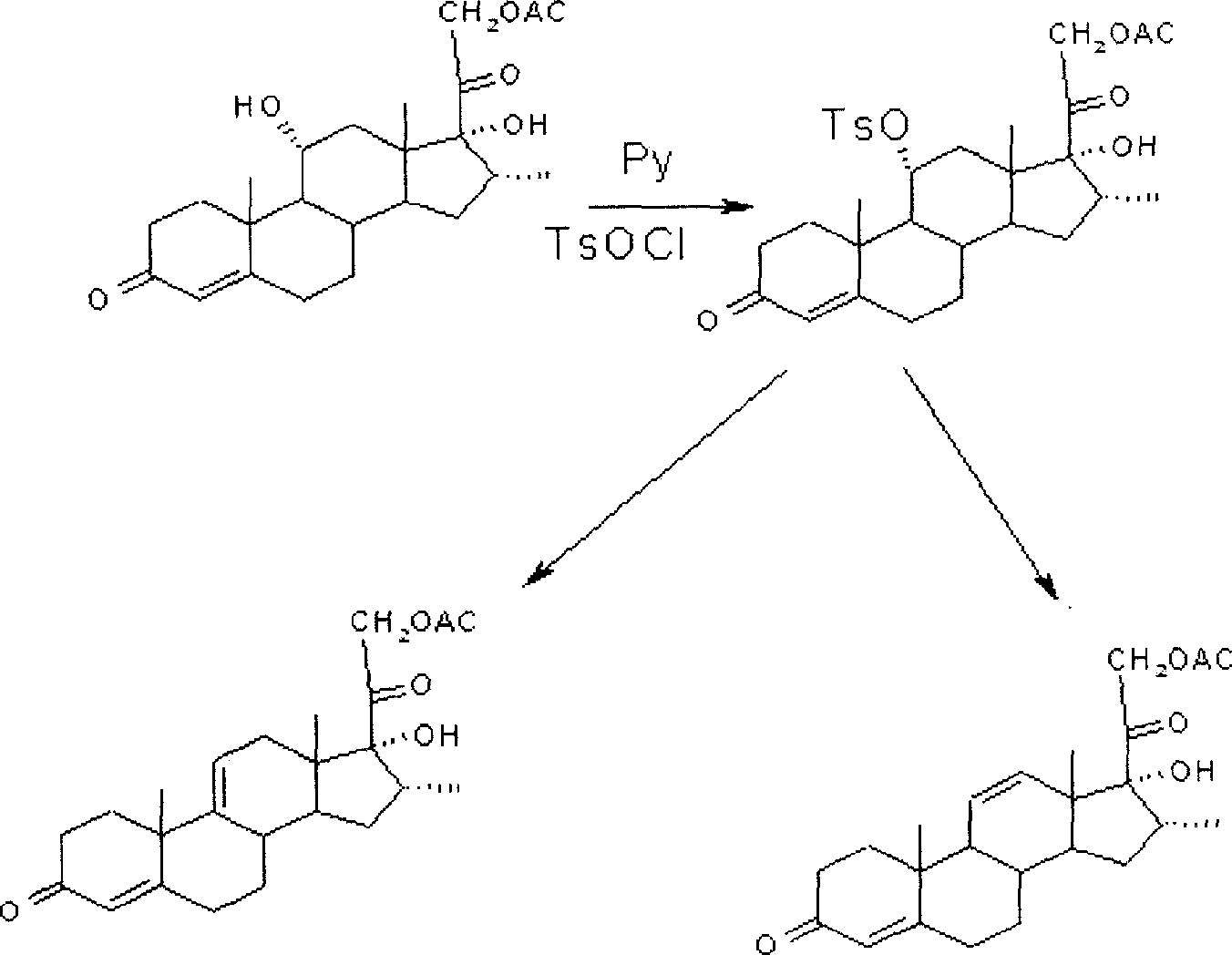
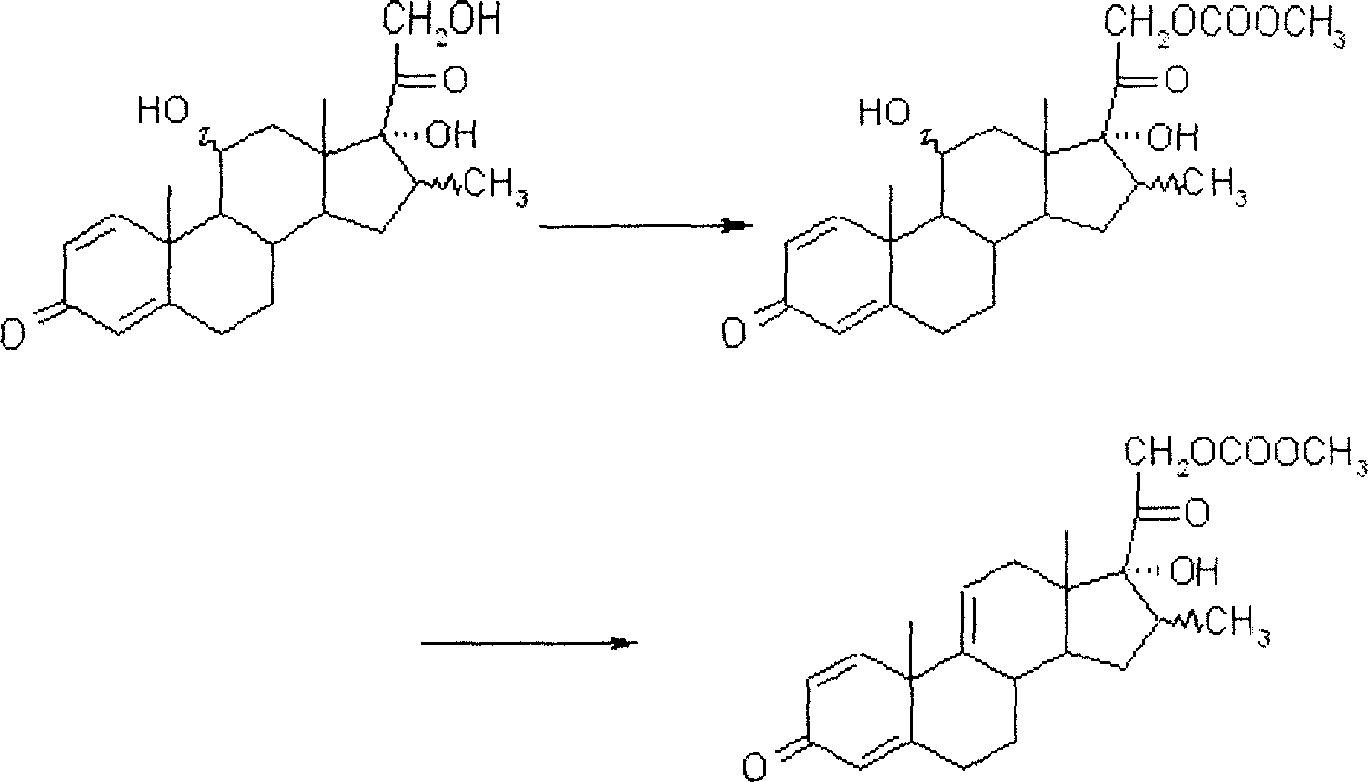
Synthesis process of a pregnane compound
A synthesis method and compound technology, applied in the field of synthesis of Δ9, pregnane steroid compounds, can solve the problems of high yield, high cost, and increased cost, and achieve the effects of high yield, low cost, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Implementation Example 1. Put 40g of 11α-hydroxyl-pregna-1,4,16-triene-3,20-dione into a three-neck flask equipped with a stirrer and a low-temperature thermometer, add 400ml tetrahydrofuran, and place in a freezer Lower the temperature to below -50°C, slowly add 40g of phosphorus pentachloride in portions, then keep the temperature at -50°C for 2 hours, then raise the temperature to 0°C, continue the reaction for 30 minutes, and dissolve the water into 1000ml of ice water, add 25% hydroxide Sodium adjusted PH = 7, stirred for 2 hours, filtered, dried, and the obtained Δ 9,11 - Pregnant compound HPLC purity 95.6%, Δ 11,12 -Isomer impurity 2.0%, yield 94.5%.
Embodiment 2
[0024] Implementation Example 2. Put 40g of 11α-hydroxyl-pregna-1,4-diene-3,20-dione into a three-neck flask equipped with a stirrer and a low-temperature thermometer, add 400ml tetrahydrofuran, and place in a freezer to cool down to Below -50°C, slowly add 40g of phosphorus pentachloride in portions, then keep the temperature at -50°C for 2 hours, then raise the temperature to 0°C, continue the reaction for 30 minutes, and water it into 1000ml of ice water, add 25% sodium hydroxide to adjust PH=7, stirred for 2 hours, filtered and dried, the obtained Δ 9,11 - Pregnant compound HPLC purity 93.6%, Δ 11,12 -Isomer impurity 2.2%, yield 94.5%.
Embodiment 3
[0025] Implementation Example 3. Put 16α-methyl-11α into a three-necked flask equipped with a stirrer and a low-temperature thermometer, 17α-dihydroxy-pregna-4-ene-3,20-dione 40g, add 400ml acetonitrile, place Cool down to -30°C in the freezer, slowly add 40ml of phosphorus trichloride dropwise, and finish adding in 1 hour, then raise the temperature to -20°C and keep it warm for 2 hours, TLC until the raw material point is less than 1%, water analysis to 1000ml of ice water, add Adjust PH=7 with 25% sodium hydroxide, stir for 2 hours, filter, and dry to obtain Δ 9,11 - Pregnant compound HPLC purity 92.3%, Δ 11,12 -Isomer impurity 3.2%, yield 90%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com