Irinotecan preparation
A technology for irinotecan preparations, which is applied in the field of irinotecan preparations and pharmaceutical compositions containing the preparations, can solve the problems of maintaining SN-38 concentration and difficulty in stabilization, so as to improve the stability of preparations and improve blood retention sexual effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
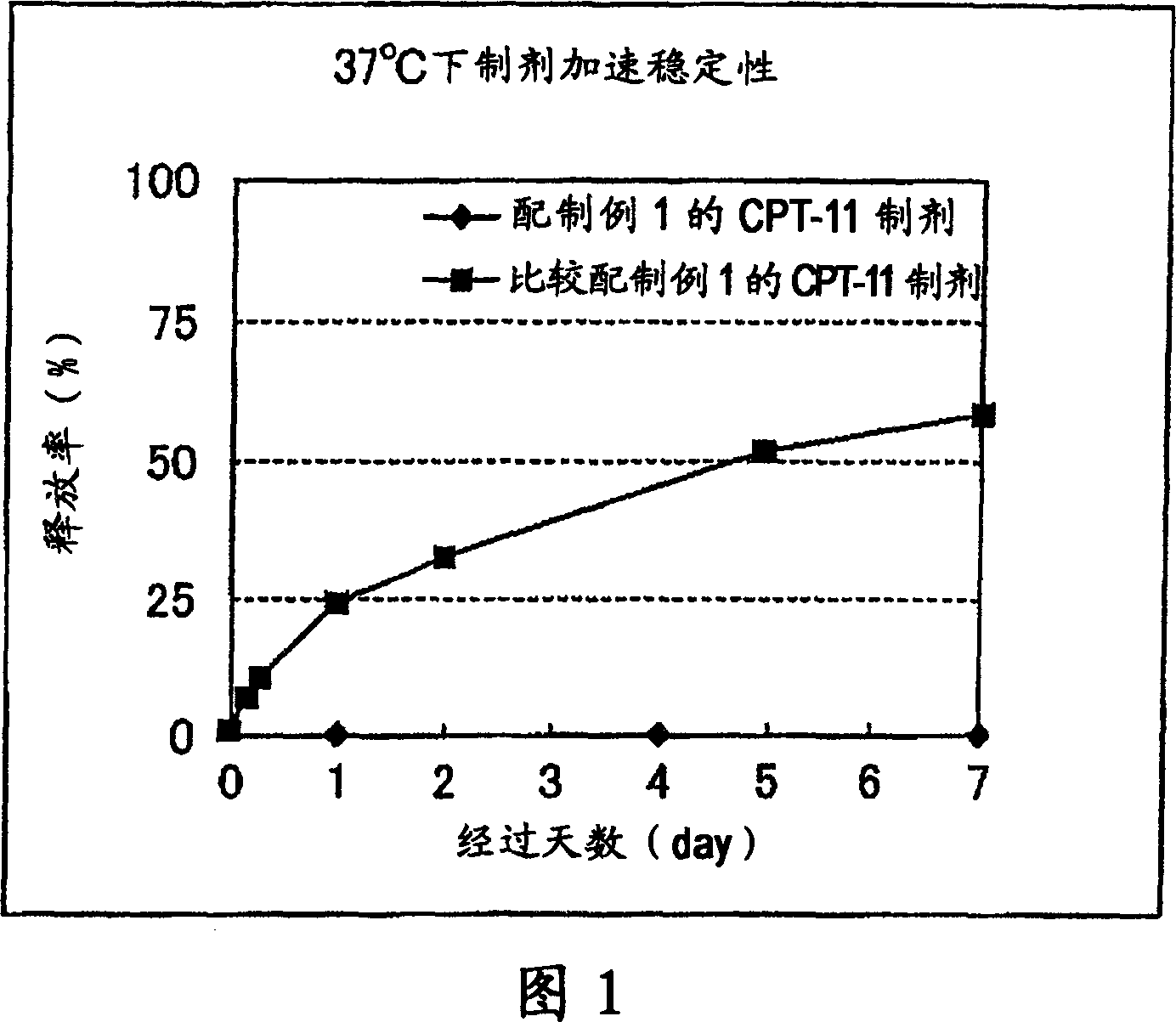
[0163] In order to confirm the realization of the high-load liposome preparation method of irinotecan hydrochloride, an attempt was made to introduce high-concentration drugs by remote filling method (preparation example 1) or passive filling method (comparative formulation example 1). Except for the different methods of introduction, all irinotecan hydrochloride loaded liposome preparations (hereinafter referred to as CPT-11 preparations) use PEG-PE (post-introduction) lipids as carriers.
[0164] (Preparation example 1)
[0165] (1) Preparation of mixed lipids: Dissolve 0.422g of hydrogenated soybean phosphatidylcholine (HSPC) and 0.176g of cholesterol (Chol) in 25ml of tert-butanol (Kanto Chemical Company) heated at 60°C, and then cool in an ice bath , Freeze-dried to prepare a mixed lipid of HSPC:Chol=54:46 (molar ratio).
[0166] (2) Preparation of liposomes: Add 10ml of 250mM ammonium sulfate solution to 0.598g of the above-prepared mixed lipids to make them fully swelled, ...
Embodiment 2
[0180] The filling amount required to obtain the highly loaded CPT-11 formulation of the present invention in the remote filling method is measured.
[0181] (Preparation example 2)
[0182] In the (4) drug embedding of Preparation Example 1, the PEG-PE prepared in the same manner as (1) to (3) was introduced into the liposome dispersion and added to the 10mg / ml CPT-11 / RO aqueous solution. The CPT-11 preparation was obtained in the same manner as in Preparation Example 1, except that the amount was changed to an amount of 0.1, 0.2, 0.4, and 0.8 at the ratio of CPT-11 / HSPC (w / w). The composition and particle size of the obtained CPT-11 formulation are shown in Table 1.
[0183] As shown in Table 1, in the remote filling method, by increasing the drug filling amount (drug / HSPC ratio), it is possible to achieve a highly loaded CPT-11 preparation with a sufficient clinical effect.
[0184] [Table 1]
[0185] Preparation example
Embodiment 3
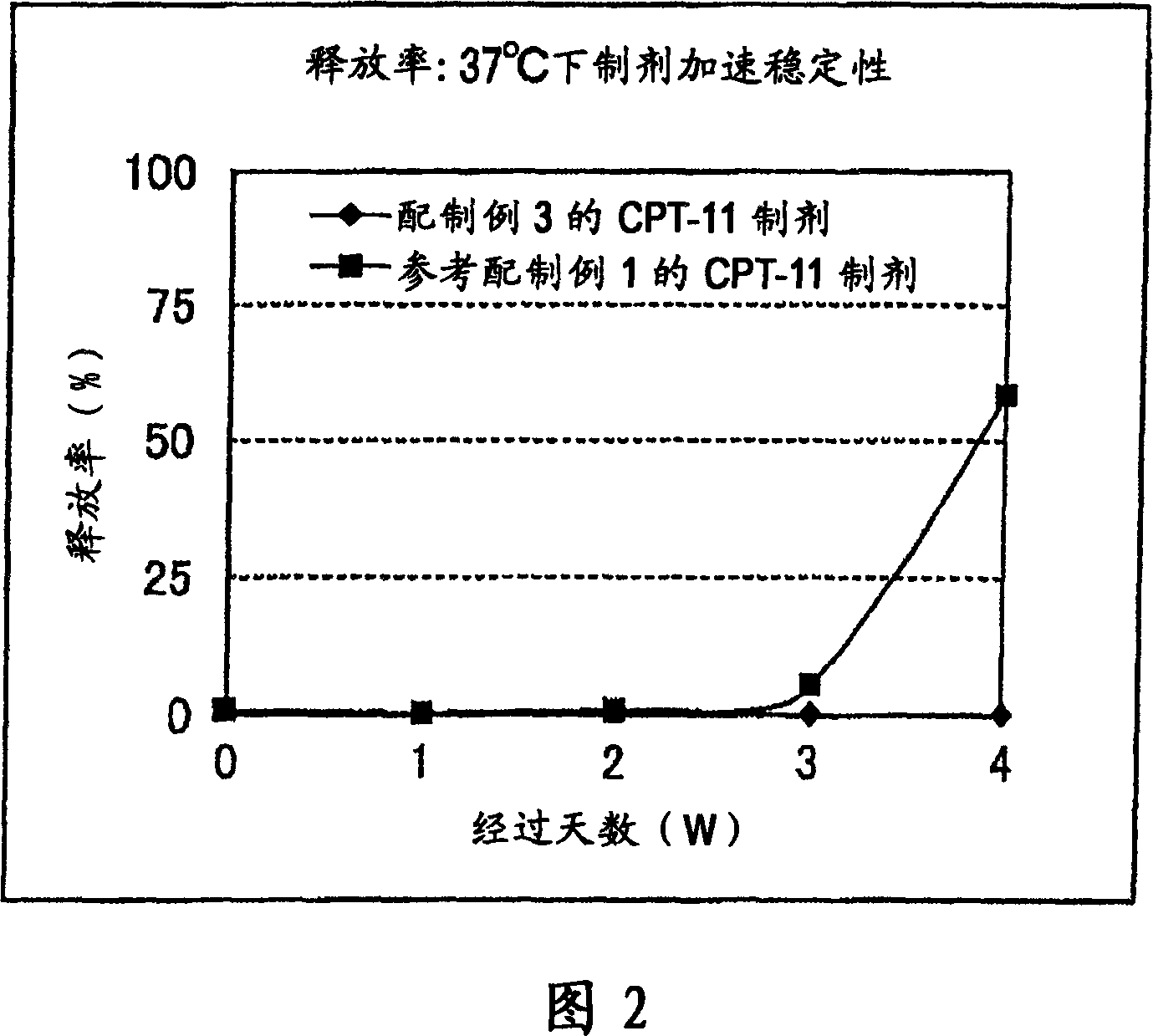
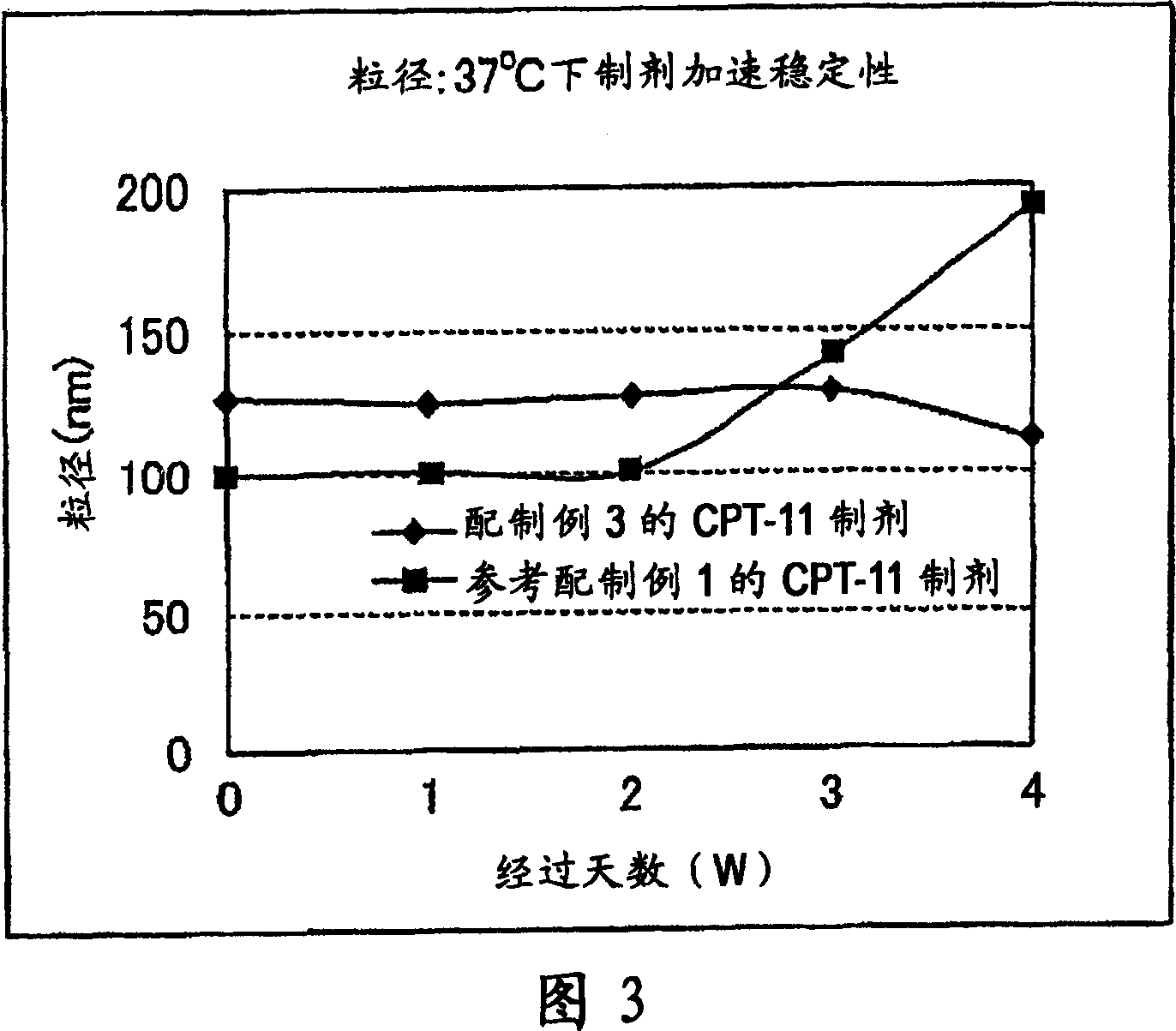
[0193] A liposome having a different membrane composition from that of Preparation Example 1 was used as a carrier to prepare a CPT-11 preparation. That is, the membrane component is PEG-PE composed of mixed lipids as shown below and then introduced into liposomes (preparation example 3) or PEG-PE is first introduced into liposomes (refer to preparation example 1), by the same as in preparation example 1. The remote filling method is used to embed irinotecan hydrochloride.
[0194] (Preparation example 3)
[0195] (1) Preparation of mixed lipids: Dissolve 1.5317g hydrogenated soybean phosphatidylcholine (HSPC), 0.6419g cholesterol (Chol) and 0.005g rhodamine dihexadecanoyl in 50mL of tert-butanol heated to 60°C After phosphatidylethanolamine (R-DHPE), it was cooled in an ice bath and freeze-dried to prepare a mixed lipid of HSPC:Chol:R-DHPE=54:46:0.1 (molar ratio).
[0196] (2) Preparation of liposomes: Except for using 0.37 g of the mixed lipids prepared above, as in Preparation ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com