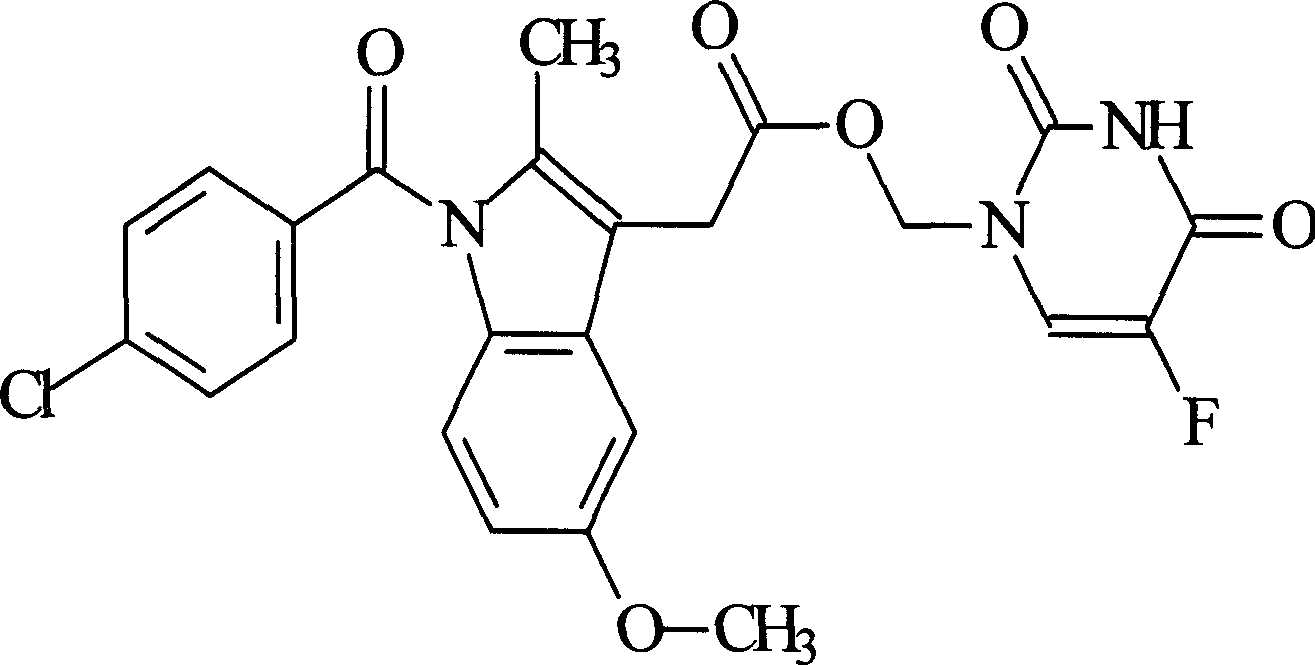
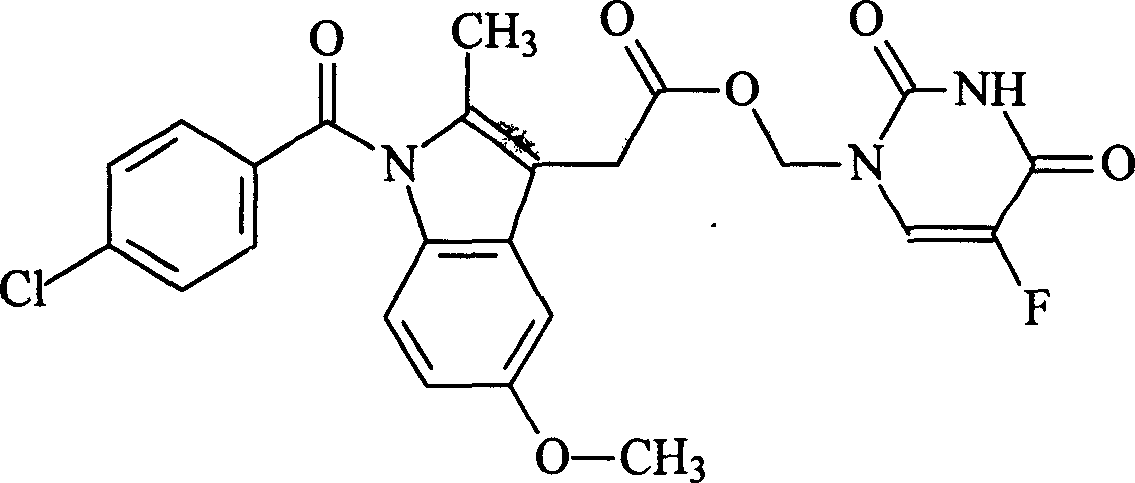
Indomethacin 5-fluorouracil methyl ester pharmaceutical compound and its formulation and preparation method
A technology of methyl fluorouracil and indomethacin, applied in the field of medicine, can solve the problems of high toxicity, restricted use and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0010] Example 1 Synthesis of indomethacin 5-fluorouracil methyl ester
[0011] 1.30g (10mmol) 5-FU and 1.78g (22mmol) formaldehyde aqueous solution (37%) were heated and stirred on a water bath at 60°C until the solids were completely dissolved, then the reaction was continued for 50min, and water and excess formaldehyde were distilled off under reduced pressure to obtain The transparent intermediate product 1,3-dimethylol-5-FU. Add 100 mL of anhydrous acetonitrile, 4.3 g (12 mmol) IDM, 2.47 g (14 mmol) N, N-dicyclohexylcarboimide (DCC), 0.08 g N, N-dimethylaminopyridine (DMAP), room temperature The reaction was stirred for more than 72 hours, and the progress of the reaction was monitored by thin layer chromatography (TLC). After the reaction was completed, the precipitate was removed by filtration, and the filtrate was distilled under reduced pressure to recover acetonitrile. The residue was dissolved in an appropriate amount of ethyl acetate, and washed successively with ...
Embodiment 2
[0017] Example 2 Antitumor activity of indomethacin-5-fluorouracil methyl ester
[0018] mouse sarcoma S 180 Tumor strain, liver cancer H 22 Tumor strains and three tumor strains of Lewis lung cancer solid tumors were used as indicators to investigate the anti-tumor activity of IFM.
[0019] In vitro resuscitation of mouse sarcoma S 180 Tumor strain, liver cancer H 22 Tumor strain and Lewis lung cancer tumor strain, cells were collected during the exponential growth phase of the cells, 1000r·min -1 Centrifuged, washed twice with PBS, sucked off the supernatant, diluted with sterile saline, adjusted to 2×10 7 pcs mL -1 . Randomly select healthy mice, inject 0.2mL of the above cell suspension into the intraperitoneal cavity, and observe the growth of the ascites of the inoculated mice. About a week after the inoculation, the abdomen of the inoculated mice is obviously enlarged and protruding, and the ascites is extracted. In a sterile test tube, dilute it with sterile sal...
Embodiment 3
[0026] Example 3 Acute toxicity of indomethacin-5-fluorouracil methyl ester.
[0027] Accurately weigh an appropriate amount of medicine, dissolve it with 1% CMC-Na to 15mL, take 6mL as the first group of medicine, dilute the remaining medicine to 15mL, take 6mL as the second group of administration, and so on. 50 mice were evenly divided into 5 groups according to sex and body weight, 10 mice in each group, and administered intragastrically according to the designed dosage, and the administration volume was 20mL / kg. Observed for 14 days after administration. The death of animals in each group was recorded. There were no obvious lesions in the major organs of the mice in the autopsy.
[0028] Bliss method calculation, 5-FU, IDM, 5-FU and IDM physical mixture 1:1 (mol / mol) and IFM half lethal dose (LD 50 ) are 82.28mg / kg, 260.09mg / kg, 200.34mg / kg and 1929.57mg / kg respectively.
[0029] The results showed that compared with the two original drugs and the physical mixture of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com