Terminal-phosphate-labeled nucleotides and methods of use
A phosphate radical and labeling technology, which is applied in the direction of chemical instruments and methods, biochemical equipment and methods, microbial determination/inspection, etc., can solve problems such as dynamic range limitation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
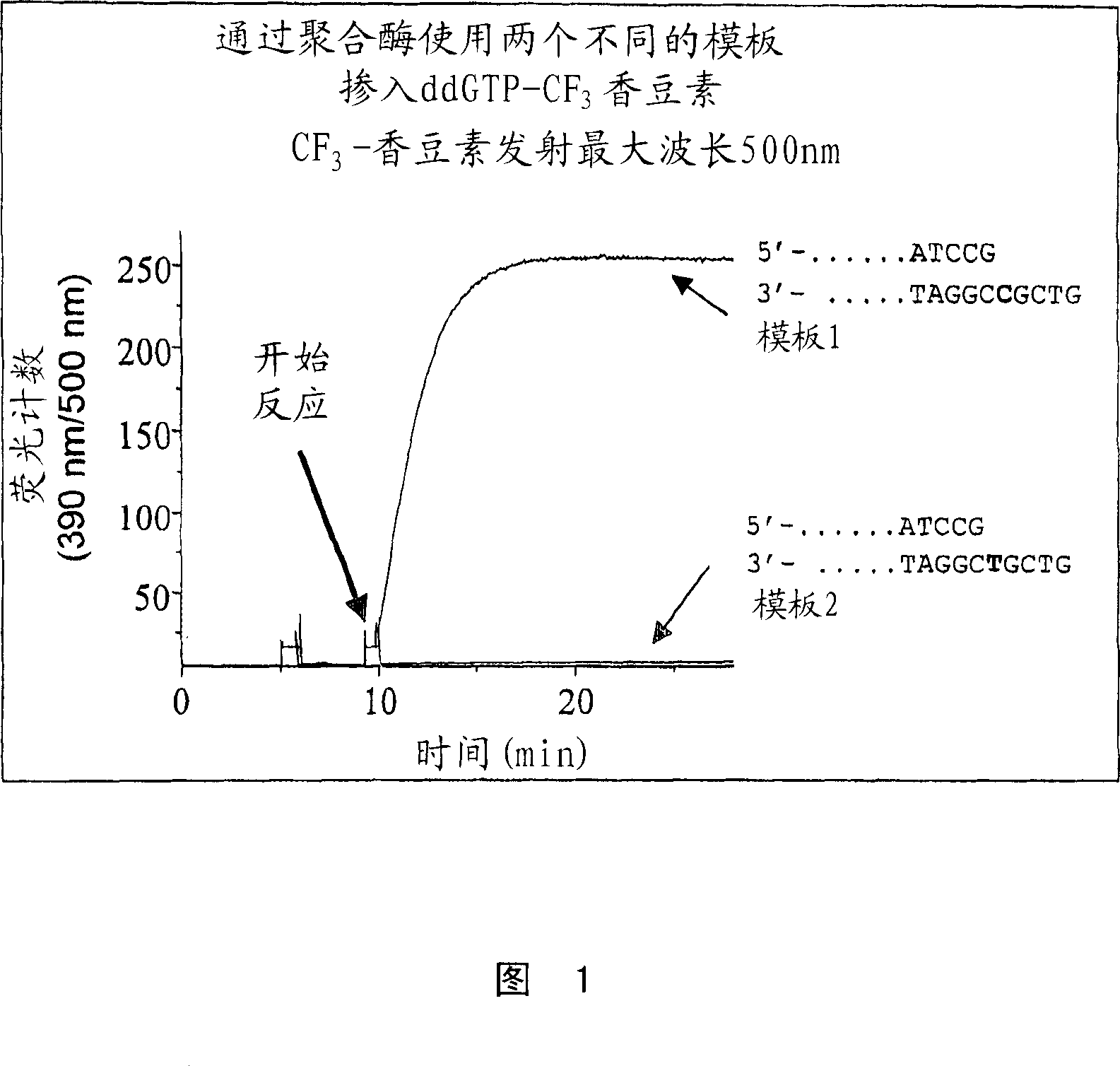
[0124] Preparation of γ-(4-trifluoromethylcoumarinyl)ddGTP (γCF3coumarin-ddGTP)
[0125] ddGTP (200 μl, 46.4mM solution, purity>96%) was co-evaporated together with anhydrous dimethylformamide (DMF, 2×0.5ml), and dicyclohexylcarbodiimide (DCC, 9.6mg, 5 eq), the mixture was co-evaporated with anhydrous DMF (0.5ml), then the residue was treated in anhydrous DMF (0.5ml), and the mixture was stirred overnight. About 20% of uncyclized triphosphate (probably from hydrolysis of the cyclic trimethylphosphate on the column) remained. Another 2 equivalents of DCC were added to the mixture, and after stirring for 2 hours, 7-hydroxy-4-trifluoromethylcoumarin (4-trifluoromethylumbelliferone, 42.7 mg, 20 equivalents) and triethylamine ( 26 μl, 20 equivalents), the mixture was stirred at room temperature, and after 2 days, HPLC (0-30% acetonitrile / 15 minutes in 0.1M triethylammonium acetate (TEAA), 30-50% acetonitrile / 5 min and 50-100% acetonitrile / 10 min, C18 3.9 x 150 mm column, flow 1 m...
Embodiment 2
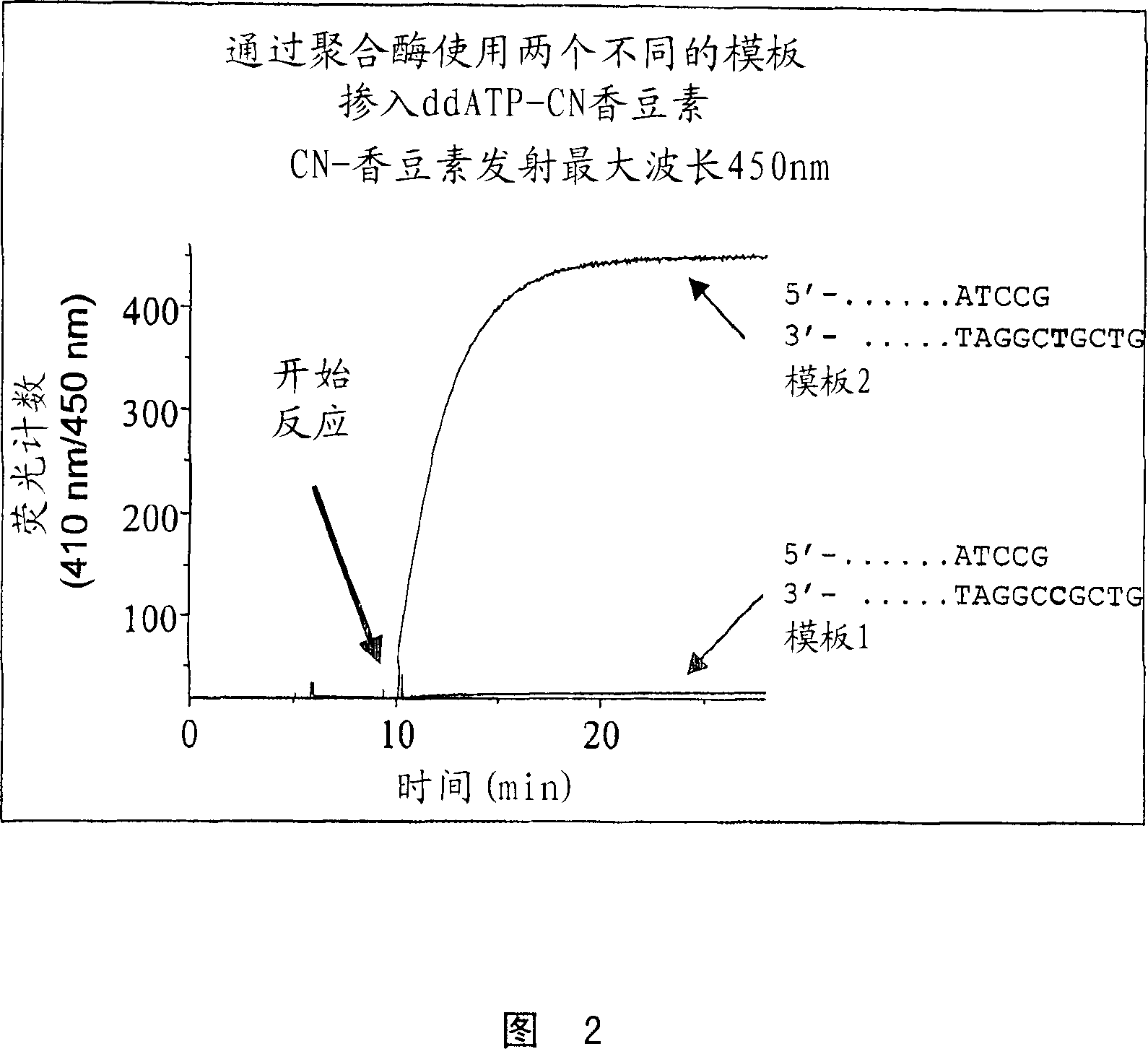
[0130] Preparation of γ-(3-cyanocoumarinyl)ddATP (γ-CNcoumarin-ddATP)
[0131]ddATP (100 μl, 89 mM solution, >96%) was co-evaporated with anhydrous DMF (2×1 ml), to which was added DCC (9.2 mg, 5 equiv), the mixture was stirred, and then co-evaporated with anhydrous DMF (1 ml), The residue was treated with anhydrous DMF (0.5ml) and 7-hydroxy-3-cyanocoumarin (33.3mg, 20eq) and TEA (25p.1, 20eq) were added after the reaction was stirred overnight at room temperature. After stirring the mixture at room temperature for 1 day a major product (55% at 254 nm) was observed at 8.1 min and another minor product was observed at 10 min (approximately 10%), no major changes were observed after one day.
[0132] The reaction mixture was concentrated on a rotary evaporator, the residue was extracted with 3 x 2 ml water and filtered, the aqueous solution was concentrated and purified on C-18 using 0-30% acetonitrile (pH 6.7) in 0.1 M TEAB / 30 min, 30 -50% acetonitrile / 10min, flow rate 15ml / mi...
Embodiment 3
[0136] Preparation of 8-9H(1,3-dichloro-9,9-dimethylacridin-2-on-7-yl)-dideoxythymidine-5′-tetraphosphate (ddT4P-DDAO)
[0137] Co-evaporate ddTTP (100gel, 80mM solution) and anhydrous dimethylformamide (DMF, 2×1ml), add dicyclohexylcarbodiimide (8.3mg. DMF (1 ml) was co-evaporated, the residue was treated with anhydrous DMF (1 ml), the reaction was stirred at room temperature overnight, HPLC showed mostly cyclized triphosphate (-82%). Concentrate the reaction mixture, wash the residue three times with anhydrous diethyl ether, redissolve in anhydrous DMF, and concentrate to dryness with a rotary evaporator. The residue was leached and stirred at 40°C for one week, HPLC showed the formation of a new product with a desirable UV profile at 11.96 minutes. (HPLC method: 0.30% acetonitrile (pH 7) in 0.1 M triethylammonium acetate / 15 min, 30-50% acetonitrile / 5 min, Novapak C-18 3.9 x 150 mm column, 1 ml / min). LCMS (ES-) also showed the main peak 834 of the M-1 peak. The reaction m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com