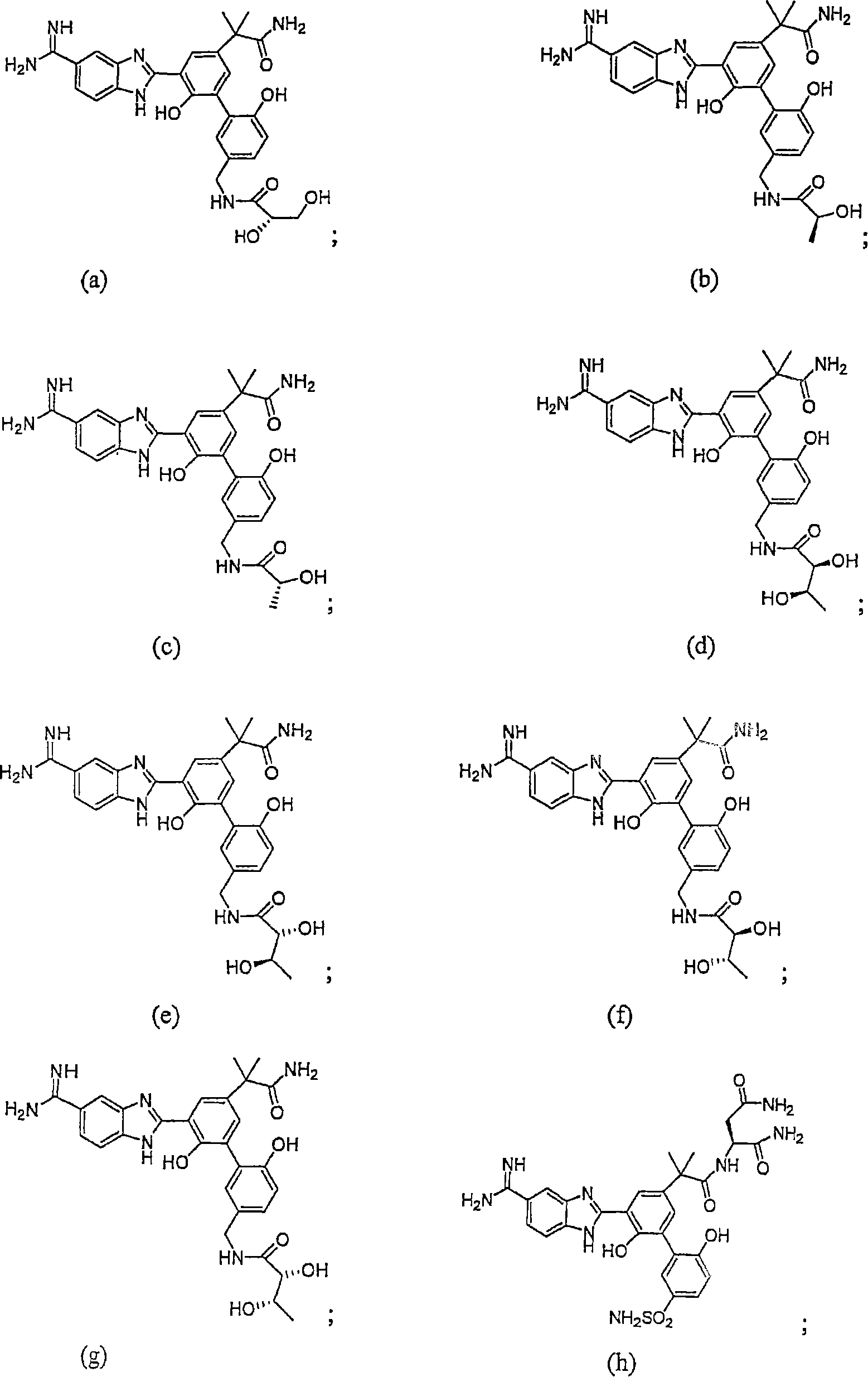
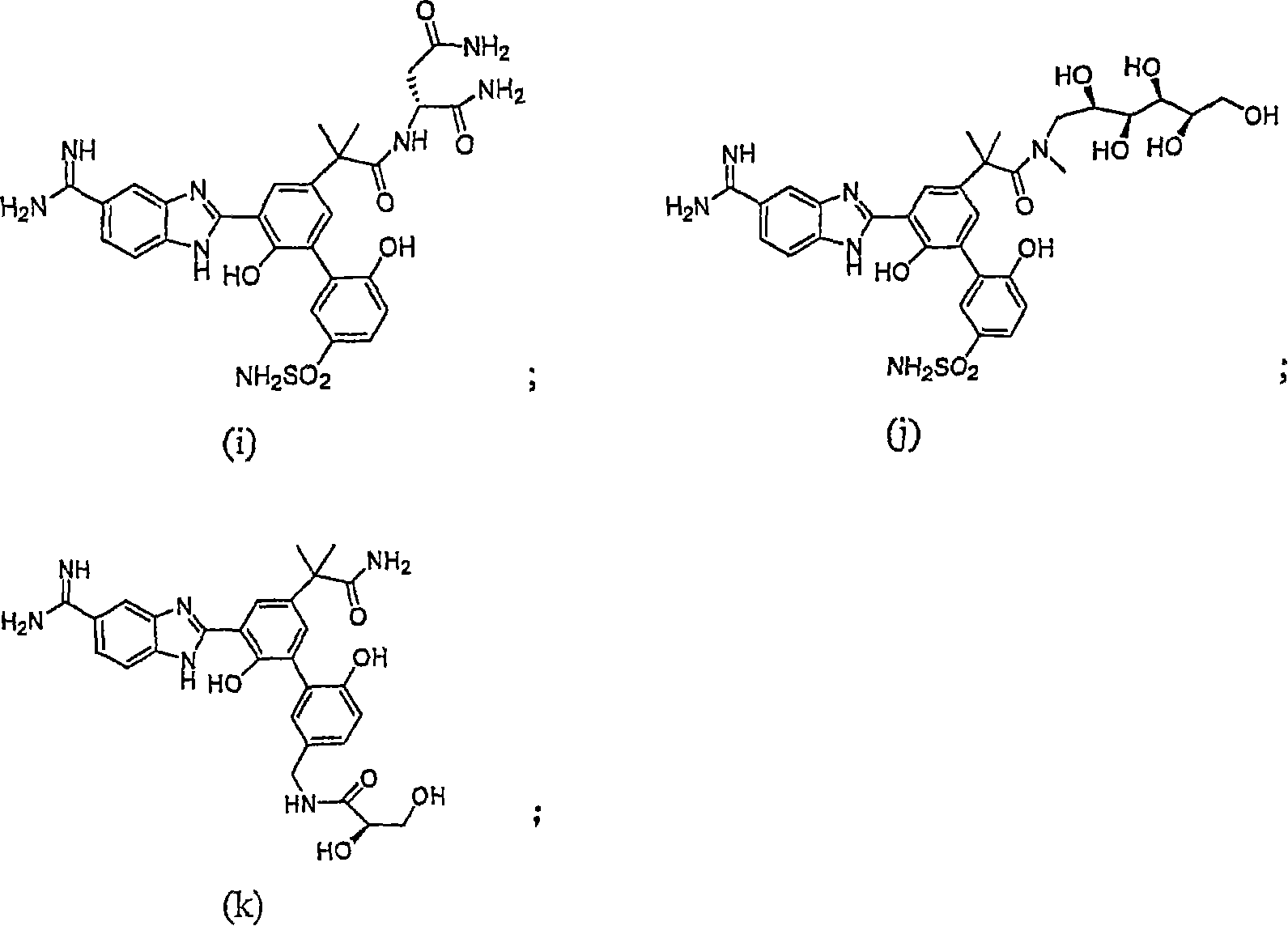
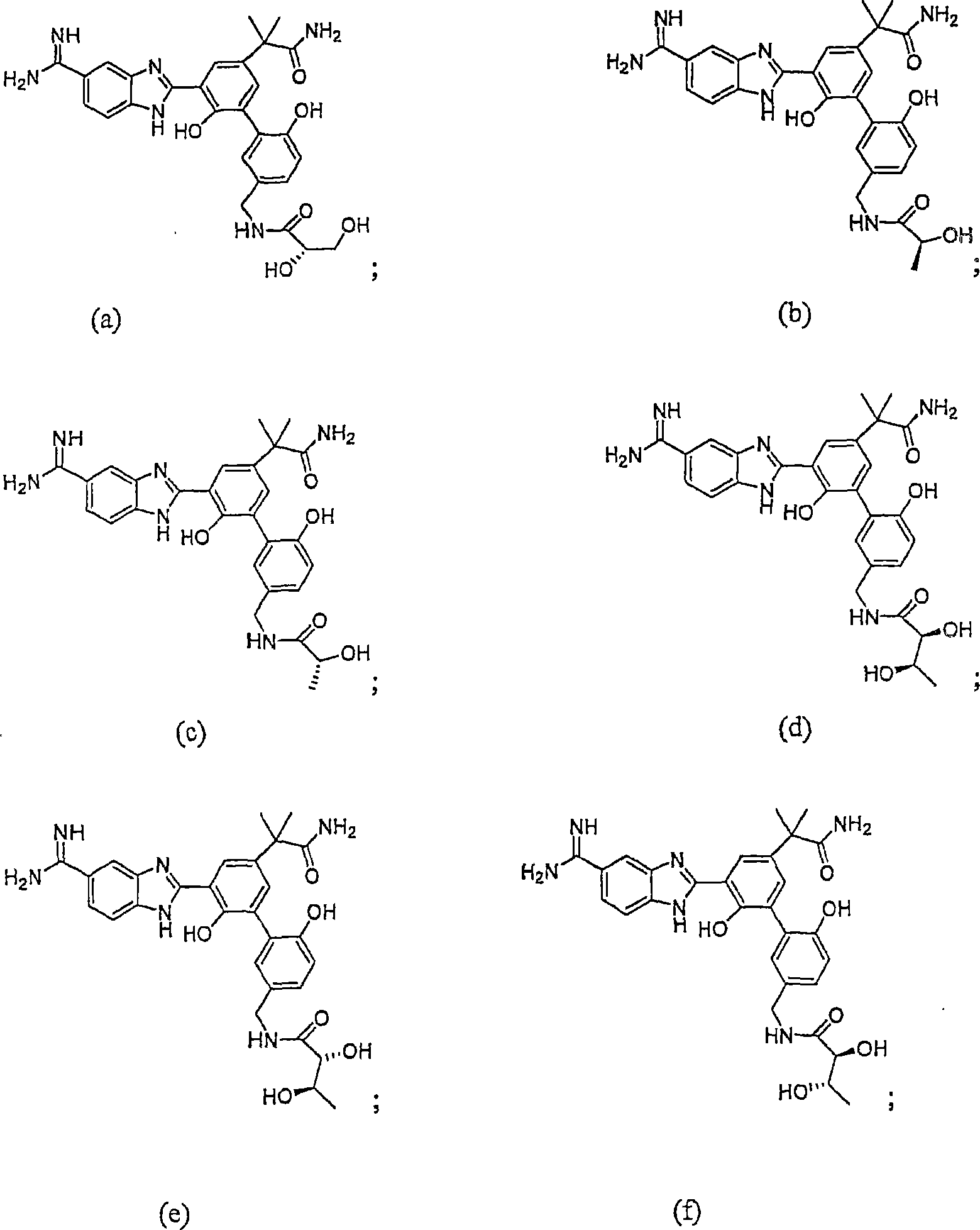
Factor VóÄa inhibitor
A technology for thrombin inhibitors and factors, applied in the field of preparing these inhibitors, treating or preventing thromboembolic diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0132] N-[3'-(5-Carboxamidino-1H-benzimidazol-2-yl)-5'-(1-carbamoyl-1-methyl-ethyl)-6,2'-dihydroxy Synthesis of biphenyl-3-ylmethyl]-(2S)-2,3-dihydroxypropionamide dihydrochloride
[0133]
[0134] step 1
[0135] To a 3-L round bottom flask equipped with a magnetic stir bar was added methyl 2-(3-bromo-5-formyl-4-hydroxyphenyl)-2-methylpropanoate (132 g, 438 mmol), potassium carbonate (66.7 g, 483 mmol) and DMF (1 L). The solution was stirred at room temperature for 0.5 hours. Iodomethane (31.5 mL, 506 mmol) was added dropwise with vigorous stirring. The reaction was complete after 3 hours. To this solution was added methyl tert-butyl ether (MTBE) (3 L) and the solution was filtered to remove inorganic salts. The solution was washed with water, then with cold 0.5% aqueous NaOH (1 L), then with brine. The aqueous layer was back extracted with MTBE (1 L). The combined organic layers were dried over sodium sulfate and concentrated. The final product can be isolated by ...
Embodiment 2
[0154] N-[3'-(5-Carboxamidino-1H-benzimidazol-2-yl)-5'-(1-carbamoyl-1-methyl-ethyl)-6,2'-dihydroxy Synthesis of biphenyl-3-ylmethyl]-(2S,3R)-2,3-dihydroxybutanamide
[0155]
[0156] step 1
[0157] (2S,3R)-2,3-O-Isopropylidene-2,3-dihydroxybutanoic acid methyl ester (5.27 g, 30.25 mmol; Fluka catalog number 59437) was dissolved in a solution containing an equimolar amount of lithium hydroxide The monohydrate (1.27 g; 30.25 mmol) was dissolved in THF / water (1:1; 220 mL) and stirred for 90 minutes. The solution was concentrated in vacuo to afford the lithium salt of (2S,3R)-2,3-O-isopropylidene-2,3-dihydroxybutanoic acid (4.90 g, 98%) as a white solid. A portion of the lithium salt of (2S,3R)-2,3-O-isopropylidene-2,3-dihydroxybutanoic acid and HATU (0.243 g; 0.64 mmol) were mixed in DMA (10 mL), then sonicated Process for 15 minutes until dissolution is achieved.
[0158] In a separate flask, 2-[5′-aminomethyl-5-(5-carbamimidino-1H-benzimidazol-2-yl)-6,2′-dihydroxybiphen...
Embodiment 3
[0162] 2S-{2-[5-(5-Formamidino-1H-benzimidazol-2-yl)-6,2'-dihydroxy-5'-sulfamoylbiphenyl-3-yl]-2- Synthesis of Methacrylamino}succinamide
[0163]
[0164] step 1
[0165] To a solution of 2-methoxy-5-tert-butylsulfamoylphenylboronic acid (4.08 g, 14.28 mmol) dissolved in methanol (36 mL) was added 2-(3-bromo-5-formyl-4-methanol oxyphenyl)-2-methylpropionate (3.0 g, 9.50 mmol) and toluene (90 mL). Potassium carbonate solution (7.14 mL, 2M, 14.28 mmol) was added and the reaction mixture was purged with nitrogen. Tetrakis(triphenylphosphine)palladium (1.10 g, 0.95 mmol) was added and the reaction mixture was refluxed for 3 hours. After cooling, the reaction mixture was partitioned with 5% citric acid solution and the organic phase was dried and evaporated. Purification by column chromatography (40% EtOAc / hexanes) afforded 2-{5'-tert-butylsulfamoyl-5-formyl-6,2'-dimethoxybiphenyl-3-yl)- 2-Methyl-propionic acid methyl ester (3.79 g, 84%).
[0166] step 2
[0167] 2-(5'-t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com