Stable cationically crosslinkable/polymerizable dental composition with a high filler content
A composition and cationic technology, applied in the direction of dentistry, dental prosthesis, dental preparations, etc., can solve the problems of scattered storage stability and no satisfactory solution, and achieve the effect of increasing the content of fillers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0445] Embodiment 1 (comparative example): the processing of filler (B-1)
[0446] a) Treatment of fillers with coupling agents (F-2) containing diol functionality:
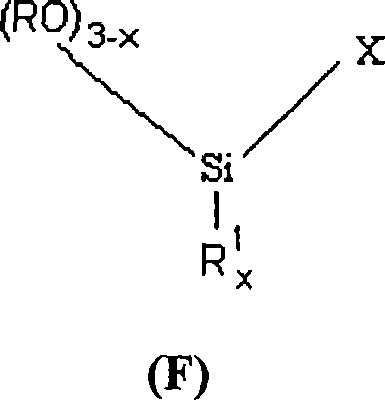
[0447] Silane glycidyloxypropyltrimethoxysilane or GLYMO shown in formula (F-1) sold by Degussa company is hydrolyzed according to the following reaction in acidic medium:
[0448]
[0449] where R = methyl
[0450] Compound (F-2) is in more or less polycondensed form and is soluble in the aqueous phase.
[0451] The solution used was a pH=3-4 solution containing 40% silane (F-2).
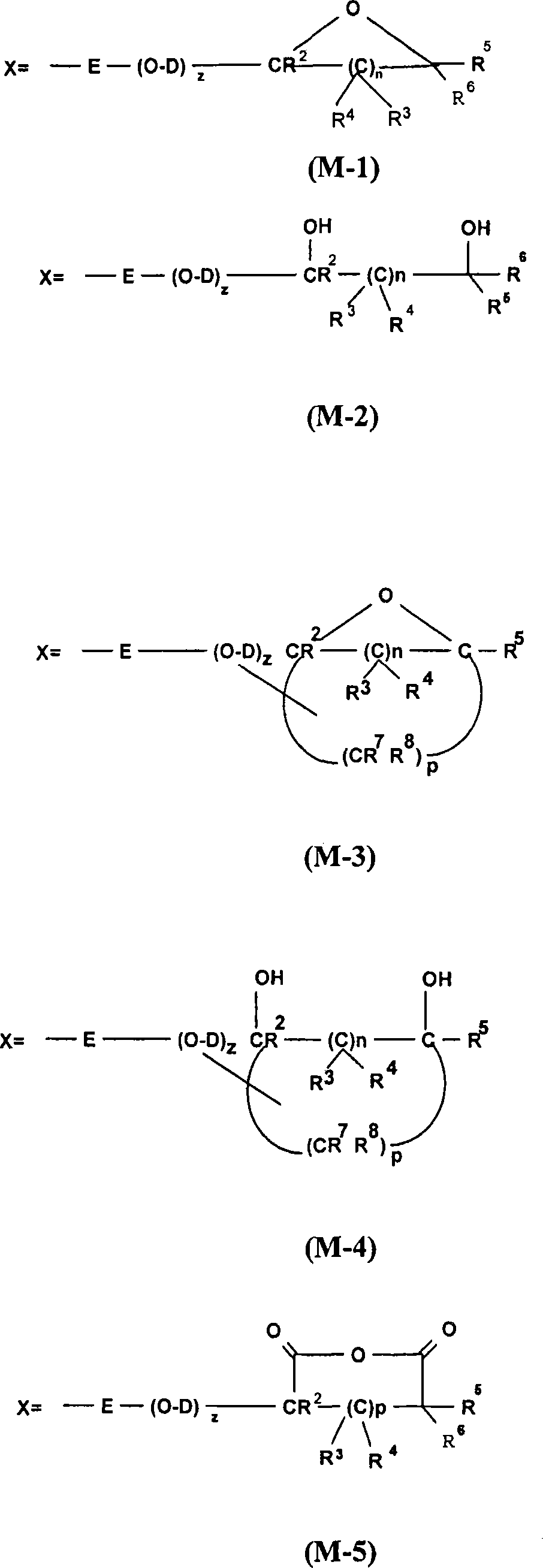
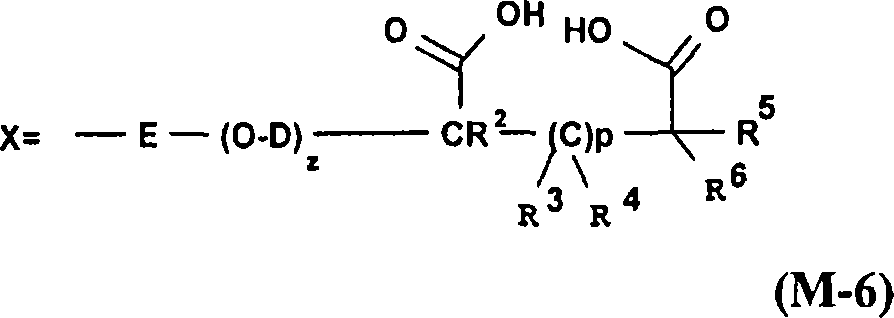
[0452] 12.5 g of this 40% silane (F-2) solution was poured into a beaker and topped up with 200 g of demineralized water. 200 g of filler (B-1) were poured into this solution and the mixture was stirred with an impeller stirrer for 1 hour at room temperature. The mixture was poured into crystallization trays, and the packing was dried in an oven at 150°C for 16 hours. The filler was then sieved through a 250 micron mesh. ...
Embodiment 2
[0469] Embodiment 2 (the present invention)
[0470] a) Treatment of the filler obtained from Example 1a) with a resin (S-1) containing oxirane functional groups
[0471] A solution containing 2.5% by weight of the oxirane-functional resin (S-1) was prepared in acetone.
[0472] 100 g of filler (B-1) treated according to example 1 a) were poured into 100 g of this 2.5% solution and stirred mechanically at room temperature for about 1 minute. The mixture was then poured into a crystallization dish and the acetone was evaporated at room temperature.
[0473] The residue was heated at 150° C. for 16 hours to polymerize the oxirane functional group-containing resin (S-1) on the surface of the filler. The filler is then screened to obtain a powder.
[0474] b) Treatment of the filler obtained from Example 1b) with a resin (S-1) containing oxirane functional groups
[0475] A solution containing 2.5% by weight of the oxirane-functional resin (S-1) was prepared in acetone. ...
Embodiment 3
[0484] Embodiment 3 (the present invention, the dual treatment of filler)
[0485] Changes in content of oxirane functional group-containing resin (S-1)
[0486] Example 2a) was repeated, varying the content of the oxirane-functional resin (S-1) during the treatment of the filler (B-1).
[0487] Dental compositions were then formulated according to Example 2c).
[0488] The specific composition corresponds to the following formula (the total filler content is 66% by weight relative to the weight of the composition):
[0489] -Resin containing oxirane functional group (S-1)---------->29.25g
[0490] - Dispersant solution containing 4% (C-1)--------->2.25g
[0491] - Photoinitiator system (P) (see Example 1):
[0492] (P-22)+(PS-31)---------->2.5g
[0493] -Ytterbium trifluoride---------->6g
[0494] -Double treated filler (B-1)---------->50g
[0495] - Untreated filler (B-3)
[0496] Combustion method silica OX50 ---------->5g
[0497] - Untreated filler (B-4) ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Flexural strength | aaaaa | aaaaa |
| Flexural modulus | aaaaa | aaaaa |
| Flexural modulus | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com