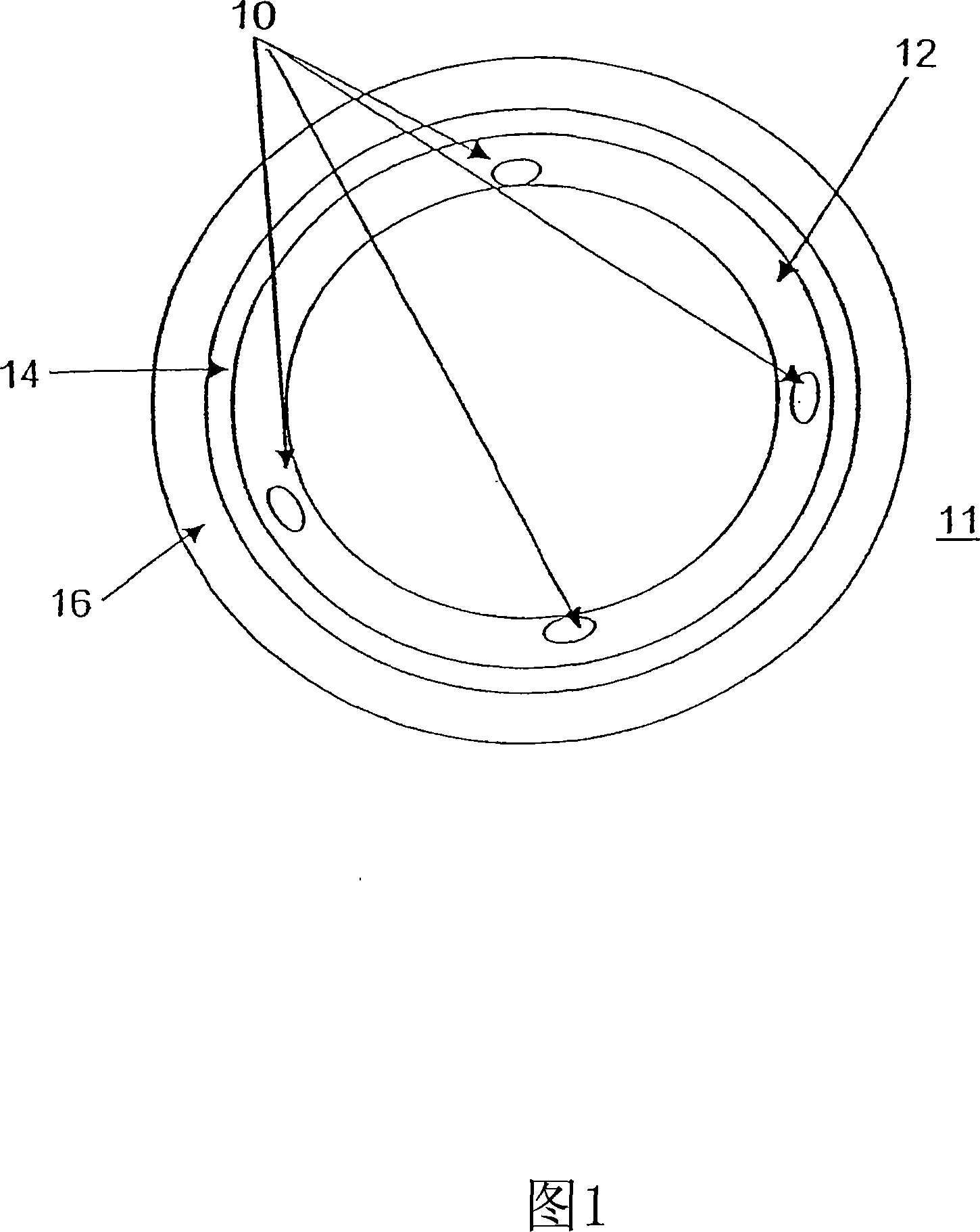
Bioactive bracket for type II diabetics and methods for use thereof
A bioactive, bioactive agent technology for use in the field of implantable stents coated with biodegradable polymers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0207] Amide Bond Formation - This example illustrates the coupling of a carboxyl group of a polymer to an amino functional group of a bioactive agent, or equivalently, a carboxyl group of a bioactive agent and an amino functional group of a polymer.
[0208]Coupling via preformed active ester; carbodiimide-mediated coupling - coupling of 4-amino-Tempo to polymer. The PEA polymer in the free carboxylic acid form was first converted to its active succinimide ester (PEA-OSu) or benzotriazole ester (PEA-OBt). This transformation can be achieved by reacting dry PEA-H polymer with N-hydroxysuccinimide (NHS) or 1-hydroxybenzotriazole (HOBt) and a suitable dehydrating agent such as dicyclohexylcarbodiimide (DCC ) in anhydrous CH 2 Cl 2 The reaction was completed at room temperature for 16 hours. After filtering off the precipitated dicyclohexylurea (DCU), the PEA-OSu product can be isolated by precipitation, or used without further purification, in which case the PEA-OSu solution ...
Embodiment 2
[0210] Ester Bond Formation - This example illustrates the coupling of carboxyl groups of a polymer to hydroxyl functional groups of a bioactive agent, or equivalently, the coupling of carboxyl groups of a bioactive agent to hydroxyl functional groups of a polymer.
[0211] Carbodiimide-mediated esterification. For coupling, samples of carboxyl-containing polymers were dissolved in DCM. To this slightly viscous solution was added a solution of hydroxyl-containing drug / biological material and DMAP in DCM. The flask was then placed in an ice bath and cooled to 0 °C. Subsequently, a solution of 1,3-diisopropylcarbodiimide (DIPC) in DCM was added, the ice bath was removed and the reaction was allowed to warm to room temperature. The coupling reaction was stirred at room temperature for 16 hours, during which time TLC was performed periodically to monitor the consumption of the hydroxyl functionality of the bioactive agent. After the indicated times, the reaction mixture was pre...
Embodiment 3
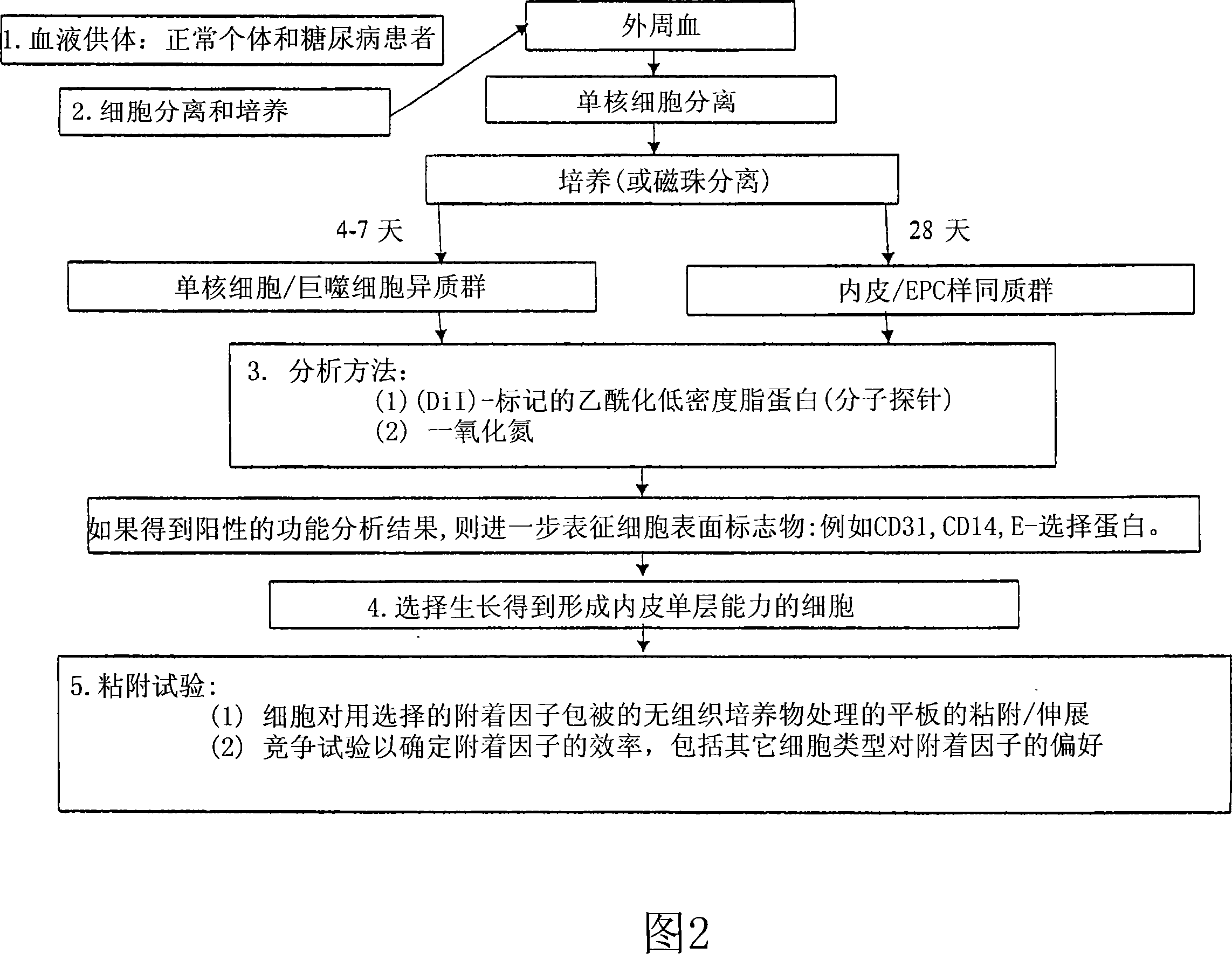
[0212] PEC isolation. To establish a protocol for isolation of endothelial progenitor cells (PECs) from peripheral blood, blood from healthy normal donors was used. Literature review lists various PEC separation schemes (J.C.I. (2000) 105: 71-77; Circ. (2003) 107: 143-149; Circ. (2003) 107: 1164-1169; Plast.Reconstruc.Surgr.( 2004) 113:284; and Am. J. Physiol. Heart Circ. Physiol. (2004) 286: H1985-H1993). Surprisingly, however, initial attempts required modification of known protocols to ensure successful separation. The flowchart in Figure 2 shows the modified protocol followed in the isolation of PECs.
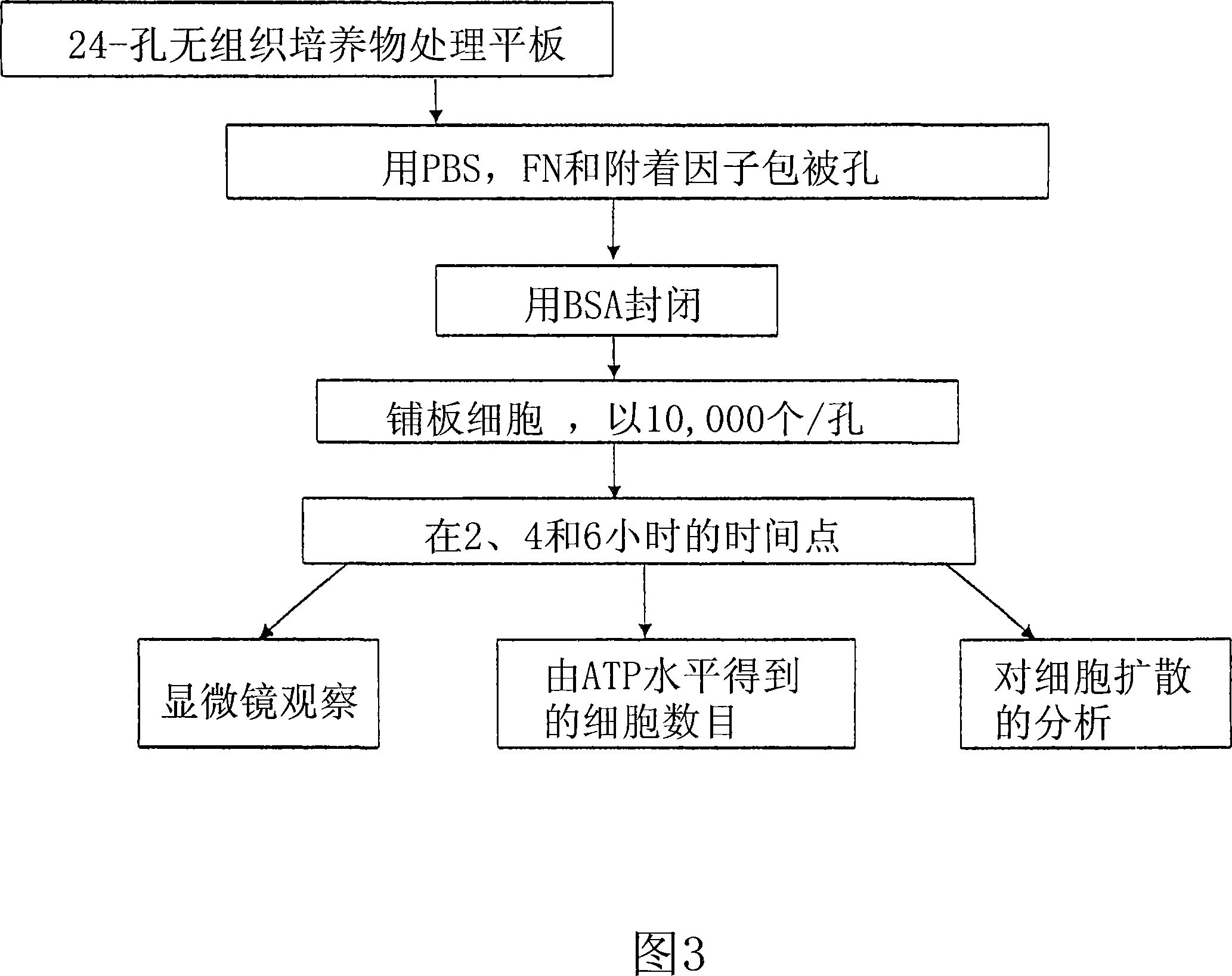
[0213] It was determined from experimental PEC isolations that cells will attach and grow better on fibronectin-coated plates than on gel-coated plates. Cells were isolated from ~120 mL of peripheral blood and an aliquot was plated on Endothelial Basal Medium and 5% FBS (Cambrex). Medium was changed every 4-5 days. The total number of cells obtained from the isolate is...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com