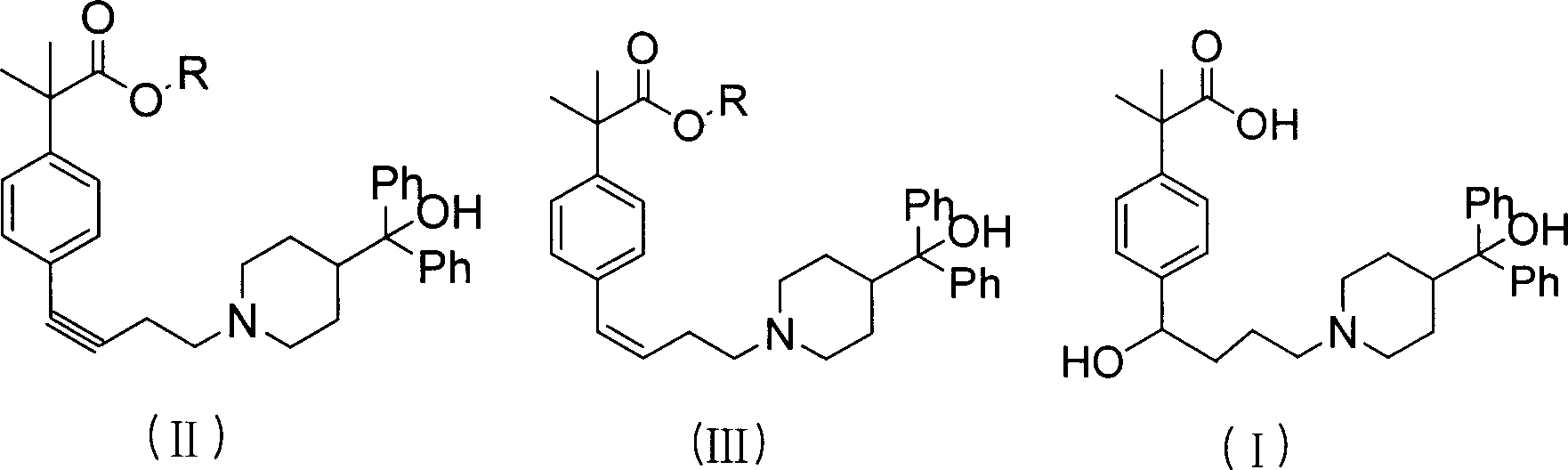
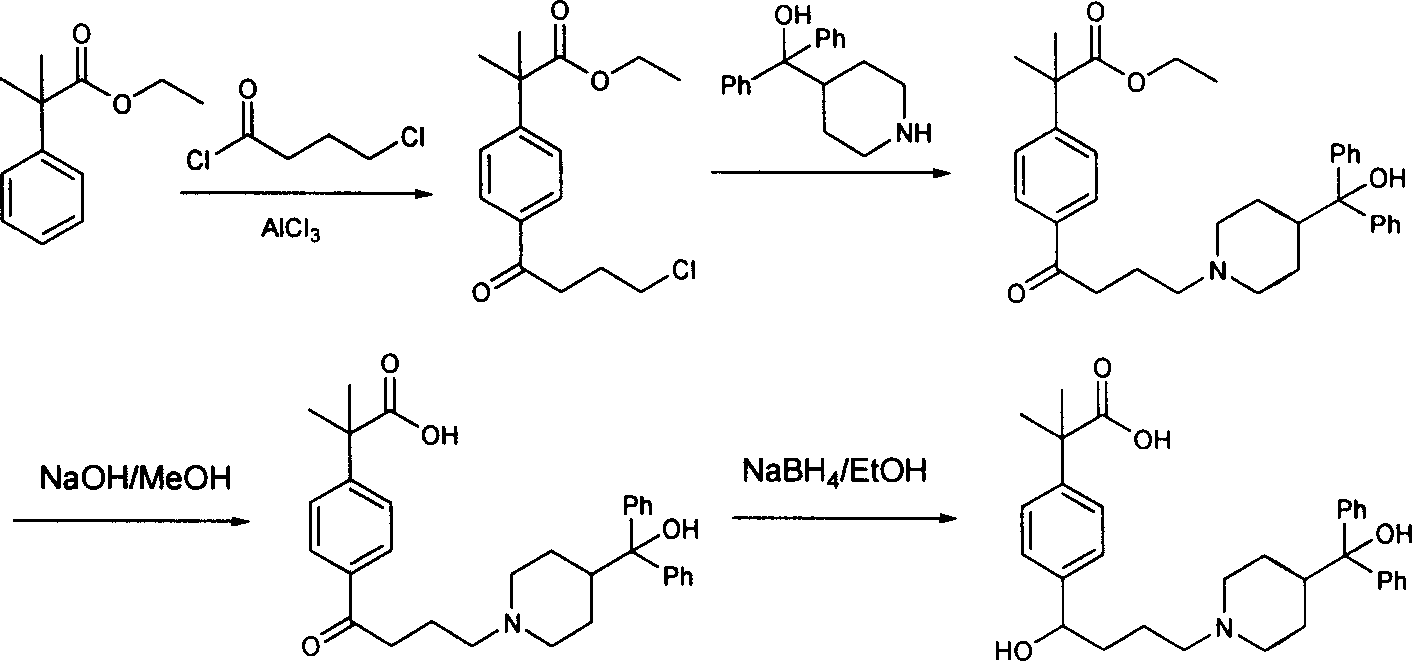
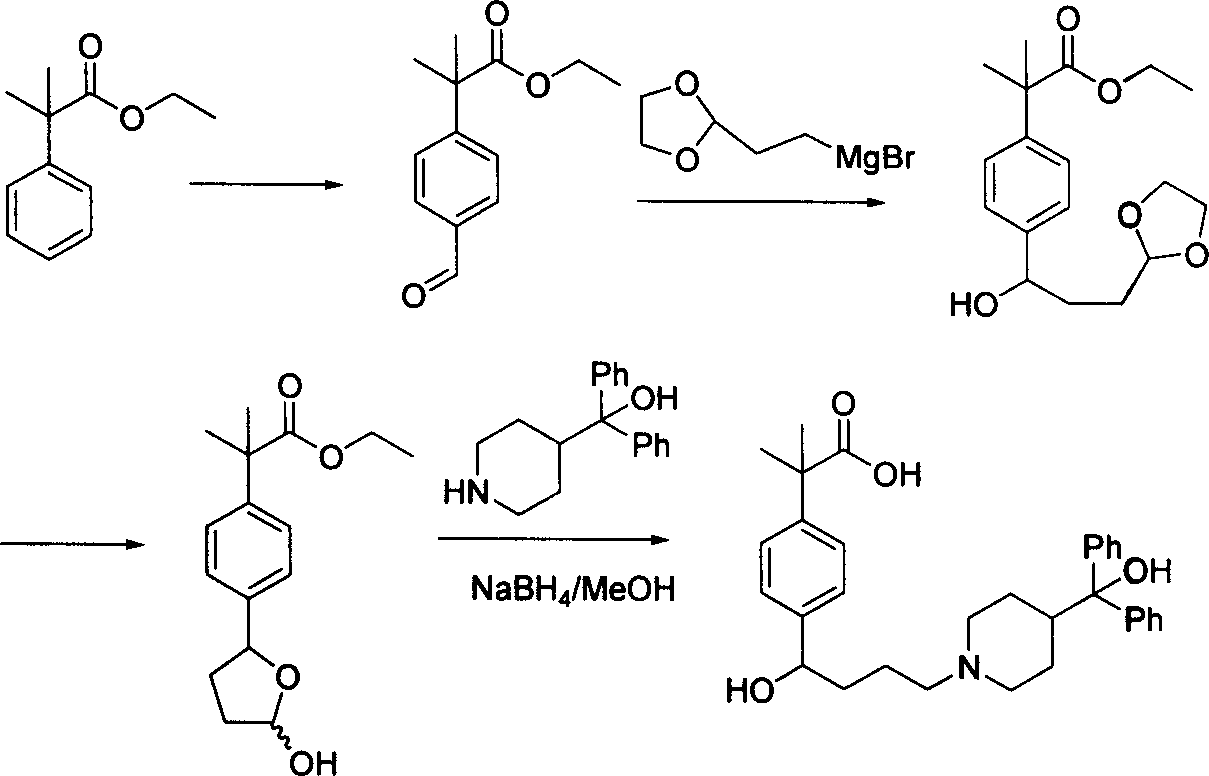
Process of synthesizing fexofenadine intermediate
A synthesis method and ligand technology, applied in the field of synthesis of fexofenadine intermediates, can solve problems such as high pressure on environmental governance, harmful to human body, difficult to recycle, etc., and achieve low environmental pollution, short reaction cycle and high product quality. Good results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1: 4-[4-[4-(hydroxyl diphenylmethyl)-1-piperidinyl]-butenyl]-α, the synthesis of α-dimethylphenylacetic acid methyl ester
[0042] Its reaction equation is:
[0043]
[0044] 4.45g (495g / mol, 8.99mmol) starting material (IV), 300ml ethanol, 0.38g 5%Pd / CaCO 3 successively added to a 500ml three-necked flask. After stirring at room temperature, 20 drops of quinoline (about 1 ml) were added dropwise. Stirring was stopped, and the air in the system was replaced with nitrogen for 3 times, and hydrogen for 1 time. Keep the hydrogen pressure at 0.02MPa, stir and react at 30°C for 10h, stop the reaction, vent, filter, concentrate and refine to obtain 4.02g of product (V). The conversion rate reaches 90%. 1 H NMR (CDCl 3 ): 7.1~7.5(aromatics, 14H), 6.40(d, 1H), 5.60(m, 1H), 3.63(s, 3H), 2.95(d, 2H), 2.41~2.52(m, 5H), 1.94~ 1.98 (m, 2H), 1.56 (s, 6H), 1.46~1.51 (m, 4H).
Embodiment 2
[0045] Example 2: (±)-4-[1-hydroxyl-4-[4-(hydroxydiphenylmethyl)-1-piperidinyl]-butyl]-a,a-dimethylphenylacetic acid methyl Synthesis of esters
[0046] Its reaction equation is:
[0047]
[0048] Compound (V) 4.02g (497g / mol, 8.09mmol), manganese acetate (0.64mmol), Schiff base ligand (0.64mmol) and NaBH 4 (16.18mmol) was added 150ml of mixed solvent of toluene and ethanol (1:1), at room temperature and normal pressure, stirred and introduced oxygen to react for 4 hours, followed by dot plate tracking, after the reaction of the reactants was complete, the ethanol was removed under reduced pressure, and the organic mixture was washed with water. phase, the organic phase was dried with magnesium sulfate, filtered and the filtrate was taken, and the solvent was removed to obtain a crude product. 3.65 g of the target product (I) was obtained by column separation with a yield of 90%. 1 H NMR (CDCl 3 ): 7.1~7.5(aromatics, 14H), 4.59(d, 1H), 3.15(d, 1H), 2.98(d, 1H), 2.40~2.5...
Embodiment 3
[0050] The reaction solvent was changed to methanol, and the others were the same as in Example 1 to obtain 3.98 g of product (V) (yield 89%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com