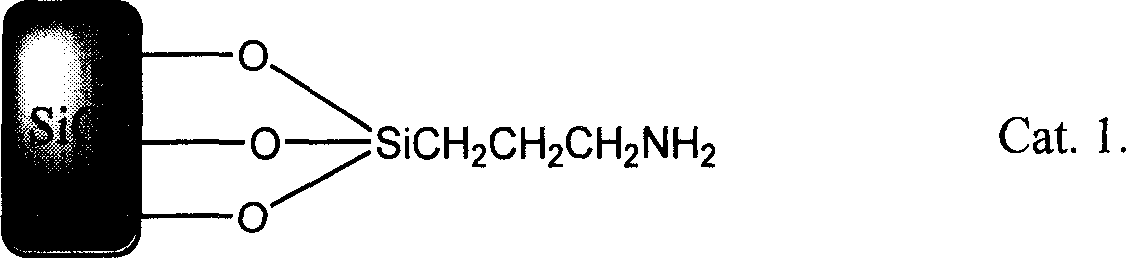Process for preparing sulfidomethyl phenol derivatives
A technology of alkyl and formaldehyde, applied in the field of antioxidant preparation, can solve the problems of easy volatility and difficult separation of dibutylamine, and achieve the effects of easy separation and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Embodiment 1: 2, the synthesis of 6-di-tert-butyl-4-((n-dodecylsulfanyl) methyl) phenol
[0023] In a 150mL three-necked flask equipped with mechanical stirring and a reflux condenser, add 20.6g (0.1mol) 2,6-di-tert-butylphenol, 4.5g (0.15mol) paraformaldehyde (relative molecular mass 240) and 20.2g (0.1mol) dodecyl mercaptan, 15g following silica-supported catalyst (Cat.) 1 (that is R in above-mentioned formula (2) 5 =O 3 Si(CH 2 ) 3 , R 6 =H)
[0024]
[0025] and 15mL of N,N-dimethylformamide, heated to 120°C, reacted for 3 hours, cooled, filtered, washed with water, and dried to obtain 41.2g of light yellow liquid with a yield of 98%. It is confirmed by analysis that the product is a compound of formula (1), wherein R 1 , R 2 For tert-butyl, respectively located in the ortho position of -OH, R 3 For n-dodecyl, -CH 2 SR 3 Located in the para position of -OH.1 HNMR analysis result shows that purity > 98%, analysis data is as follows:
[0026] 1 HNMR (CD...
Embodiment 2
[0027] Embodiment 2: the synthesis of 2-(2-hydroxyethylthio)methylphenol
[0028] In a 150mL three-necked flask equipped with mechanical stirring and a reflux condenser, add 9.4g (0.1mol) of phenol, 4.5g (0.15mol) of paraformaldehyde (relative molecular mass 240) and 7.8g (0.1mol) of mercaptoethanol , and 10 g of silica-supported catalyst 1 (that is, R in the above formula (2) 5 =O 3 Si(CH 2 ) 3 ,R 6 =H), 12g (0.1mol), and 15mL of N,N-dimethylformamide, heated to 155°C, reacted for 1 hour, cooled, filtered, washed with water, and dried to obtain 41.2g of light yellow liquid with a yield of 98% . It is confirmed by analysis that the product is a compound of formula (1), wherein R 1 ,R 2 is H, located at the ortho and para positions of -OH, R 3 for -CH 2 CH 2 OH, -CH 2 SR 3 Ortho to -OH. The analysis data is as follows:
[0029] 1 HNMR (CDCl 3 ): 7.25-7.10 (m, 2H, C 6 H 4 ), 6.85-6.70 (m, 2H, C 6 H 4 ), 6.65-6.00 (br, 1H, Ph-O H ), 3.90 (s, 2H, PhC H 2 S...
Embodiment 3
[0030] Example 3: Synthesis of 2,6-di-tert-butyl-4-((n-dodecylthio)methyl)phenol
[0031] In a 150mL three-necked flask equipped with mechanical stirring and a reflux condenser, add 20.6g (0.1mol) 2,6-di-tert-butylphenol, 4.5g (0.15mol) paraformaldehyde, 20.2g (0.1mol) Dodecyl mercaptan, 8g following catalyst 2 supported by polystyrene (i.e. R in the formula (2) 5 CH 2 ,R 6 =H)
[0032]
[0033] Replacing the catalyst supported by silica (1) Other conditions are the same as in Example 1. Heated to 120° C., reacted for 3 hours, cooled, filtered, washed with water, and dried to obtain 39.8 g of light yellow liquid. The product was the same as in Example 1, with a yield of 94.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com