Stable pharmaceutical composition of fluoroether compound for anesthetic use, method for stabilizing a fluoroether compound, use of stabilizer agent for precluding the degradation of a fluoroether com
A stabilizer and composition technology, applied in the field of preventing the degradation of fluoroether compounds and sevoflurane, can solve the problems of unproven and insufficient inhibition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] Example 1. Degradation of sevoflurane by acidic substances.
[0075] The purpose of this pilot study was to select stress conditions for the following studies using stabilizer substances.
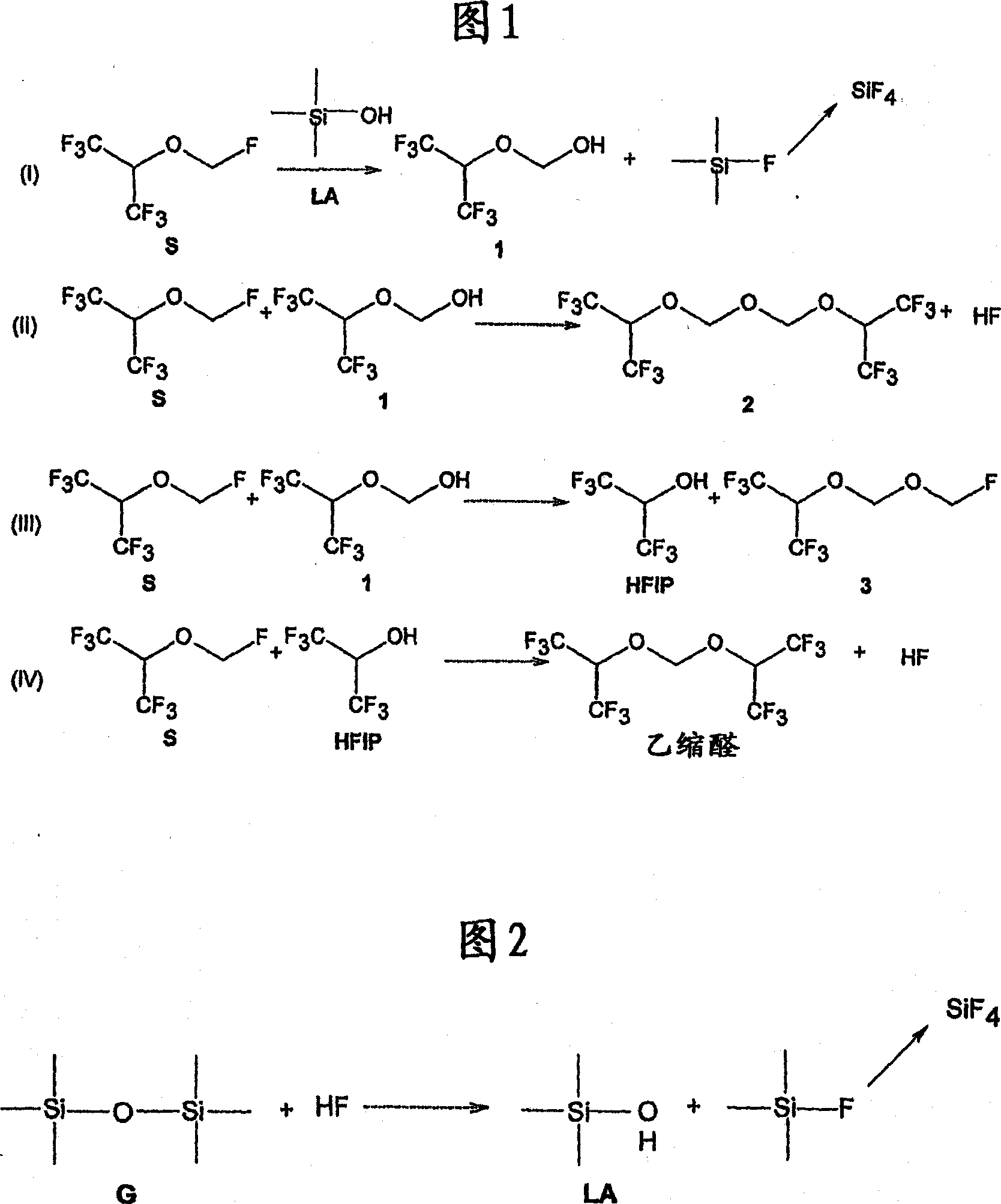
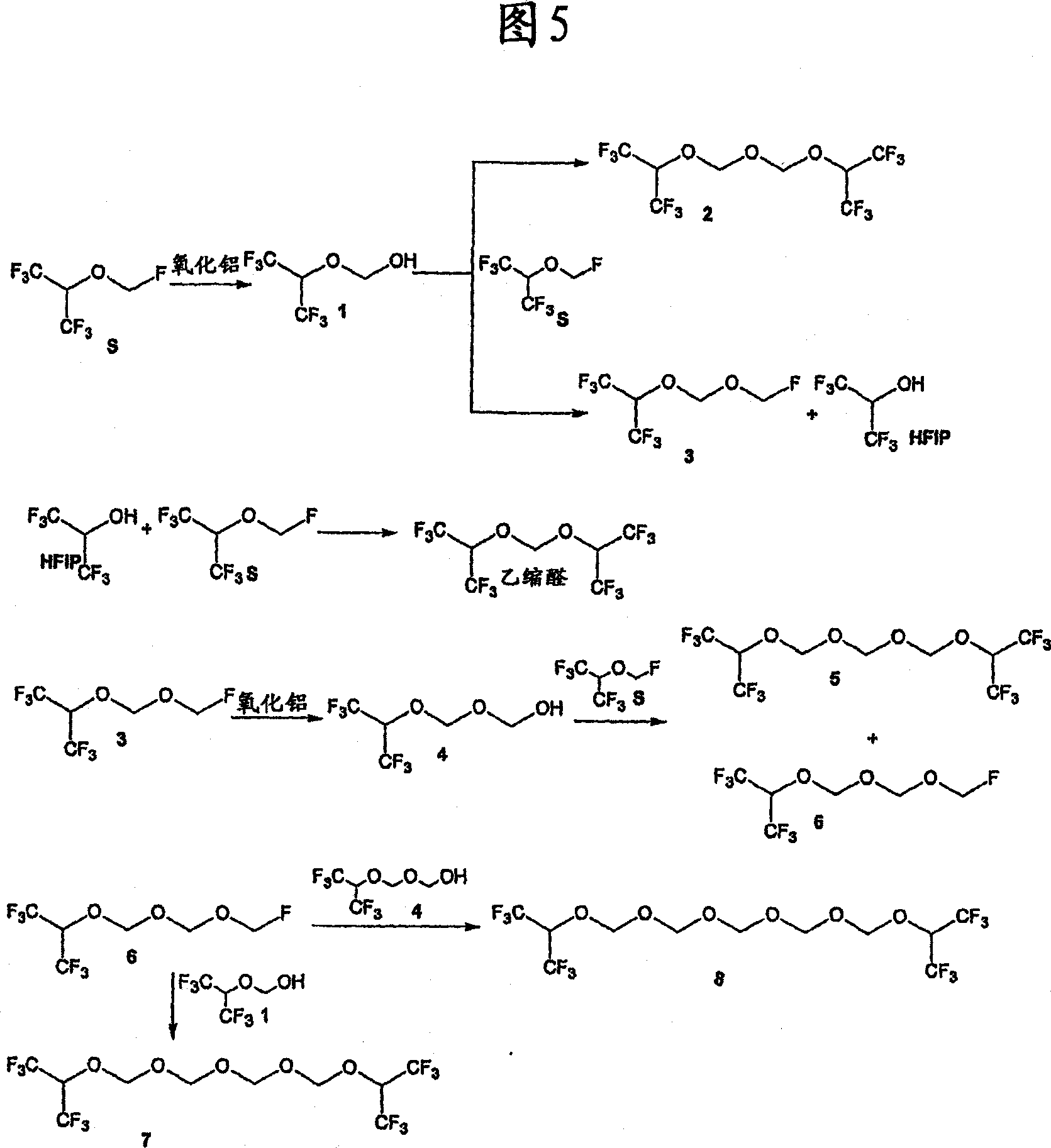
[0076] For example, when the anhydrous sevoflurane sample was mixed with alumina (Al 2 o 3 ) and heated at 60°C for 22 hours, the degradation of sevoflurane by acidic substances can be observed.
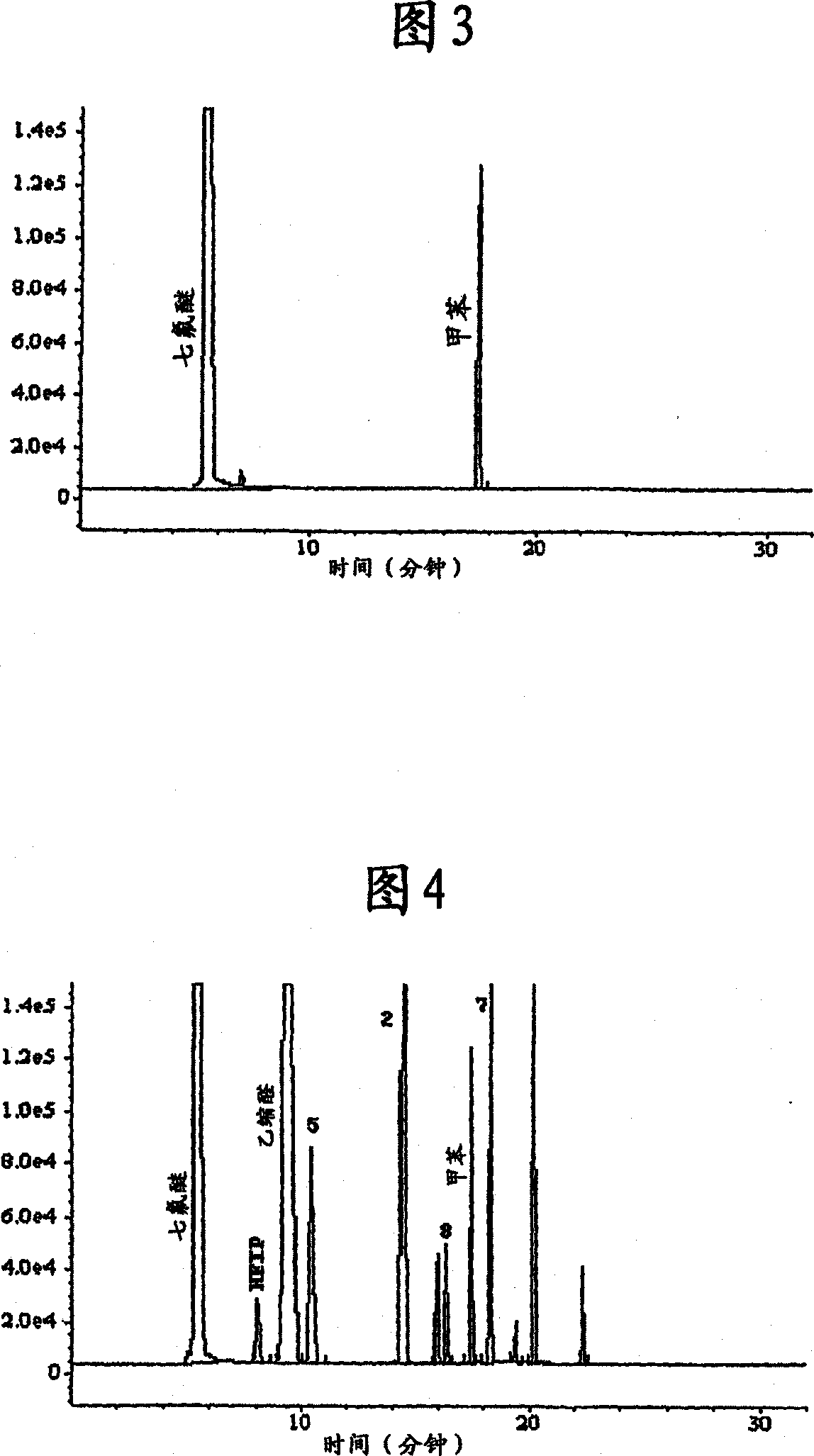
[0077] The sevoflurane used for this test was previously dried over molecular sieves to a moisture content of 20 ppm. Add 20 mL of anhydrous sevoflurane to two type III glass bottles with a capacity of 100 mL, and add 20 mg of aluminum oxide to one of the bottles, finally reaching 1.0 mg Al per mL of sevoflurane 2 o 3 . Both bottles were sealed with stoppers and screw metal caps and heated in an oven at 60°C for 22 hours. After this period, samples were analyzed in duplicate by gas chromatography using internal standard addition (toluene). Figure 3 shows the chromatogram of an anhydr...
Embodiment 2
[0080] Example 2. Effect of water on the stability of sevoflurane.
[0081] This example shows a study on the effect of water on the stability of sevoflurane. According to document WO 98 / 32430, a water content of 150 ppm to 1400 ppm present in sevoflurane will ensure its stability against the formation of degradation products.
[0082] The study was performed using sevoflurane dried with molecular sieves to achieve an initial moisture content of 20 ppm. In the presence of water, the degree of protection or degradation of sevoflurane was evaluated from sevoflurane samples containing different water contents, treated with or without alumina, where the treatment was carried out at 1 mg alumina per ml sevoflurane ratio is carried out. Samples were prepared and placed in Type III glass vials, and the vials were sealed with stoppers and screw metal caps.
[0083] The samples were placed under two stress conditions, one cycle of heating in an oven at 60°C for 22 hours, and another...
Embodiment 3
[0090] Example 3. Adding polyols or saturated cyclic alcohols to stabilize sevoflurane against the degradation of aluminum oxide.
[0091] In this example, polyols and saturated cyclic alcohols were used to prevent the degradation of sevoflurane by alumina. The substances selected in each group were propylene glycol and menthol, respectively.
[0092] Samples were prepared containing 0, 50, 200, 600, 1000 and 1400 ppm stabilizer. The sevoflurane used to prepare these samples was previously dried over molecular sieves to obtain a water content of 20 ppm. The study consisted of exposing sevoflurane to an acidic substance, subjecting the samples to stress by heating at 60 °C for 22 h, and evaluating the chromatographic purity of sevoflurane after stress by comparison. Activated alumina was used as the acidic substance at a constant dosage of 1 mg activated alumina per ml of sevoflurane.
[0093] 20 mL of test sevoflurane containing defined amounts of stabilizers (0, 50, 200, 6...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com