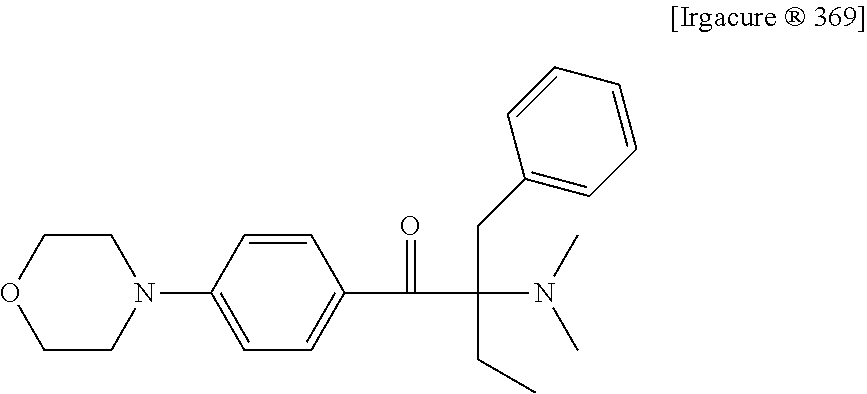
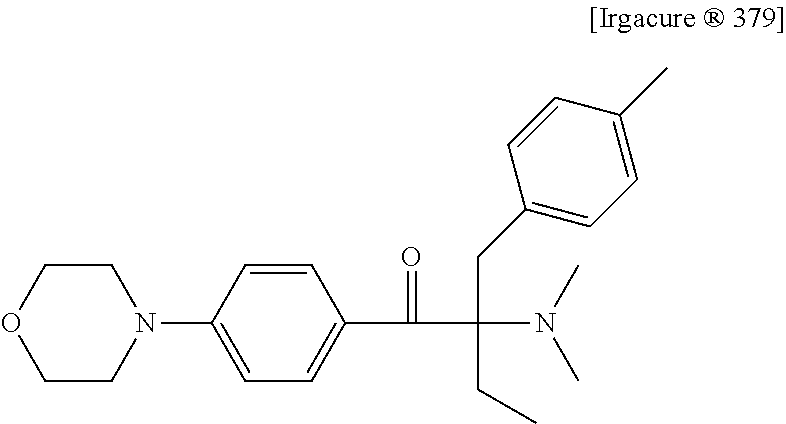
Liquid photo initiating compound and uses of the same
a technology of initiating compound and liquid photo, which is applied in the field of liquid photo initiating compound, can solve the problems of poor solubility of most UV inks, prone to precipitation of irgacure® 369, and require additional grinding and heating processes, and achieve the effect of improving all the aforementioned defects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
on of Liquid Photo Initiating Compound Represented by Formula Ia
[0058]34 g 2-(dimethylamino)-1-[4-[(2-hydroxyethyl)amino]phenyl]-2-(phenylmethyl)-1-butanone (CAS 862589-48-8); the synthesis method of it may refer to TW 1277834), 13.0 g 2,2-dimethoxybutane (CAS 3453-99-4), 0.17 g PTSA (p-toluenesulfonic acid), and 100 mL toluene were added in sequence to a 250 mL three-necked flask at room temperature and then the reaction as shown below was carried out at reflux. The by-product methanol produced by this reaction was removed by Dean-Stark evaporator.
[0059]
[0060]The reaction was monitored by High Performance Liquid Chromatography (HPLC). After the reaction was completed, the reaction product was cooled to room temperature and washed with 17 g pure water for three times. The organic layer was collected and then concentrated under vacuum to obtain the compound represented by Formula Ia (hereinafter “photo initiating compound Ia”) as a yellowish and viscous liquid. The yield is 95%.
[0061...
example 2
on of a Liquid Photo Initiating Compound Represented by Formula Ib
[0063]34 g 2-(dimethylamino)-1-[4-[((2-hydroxyethyl)amino]phenyl]-2-(phenylmethyl)-1-butanone, 12.0 g 2,2-dimethoxypropane (CAS 77-76-9), 0.17 g PTSA (p-toluenesulfonic acid), and 100 mL toluene were added to a 250 mL three-necked flask at room temperature in sequence and the reaction as shown below was carried out at a condition the same as the previous example.
[0064]
[0065]The reaction was monitored by High Performance Liquid Chromatography (HPLC). After the reaction was completed, the reaction product was extracted with 17 g pure water for three times. The organic layer was collected and then concentrated under vacuum to obtain the compound represented by Formula Ib (hereinafter referred to as “photo initiating compound Ib”) as a yellowish and viscous liquid. The yield is 93%.
[0066]The photo initiating compound Ib was subjected to nuclear magnetic resonance analysis, and the results are as follows:
[0067]
Nuclear 1H N...
example 3
y Test
[0068]Mixtures I to III were prepared according to the composition provided below, and then 6 parts by weight of photoinitiator Trgacure® 369 (available from IGM), photoinitiator R-gen® 919 (available from Chitec Technology Co., Ltd.) or photo initiating compound Ia was added to each of the mixtures I to III. The obtained mixtures were ultrasonicated for 1 hour at room temperature, and the dissolution of each of Irgacure® 369, R-gen® 919 and photo initiating compound Ia was observed and described in the following Table 1.
[0069]
Mixture I: a mixture of 50 parts by weight of 1,6-hexanediol diacrylate (product name: EM221, Eternal MaterialsCo., Ltd.) and 50 parts by weight of polyestertetraacrylate (product name: Oligomer 6325-100, Eternal Materials Co., Ltd.) with a viscosity of 41.2 cPMixture II:a mixture of 50 parts by weight of trimethylolpropane triacrylate (product name: EM231, Eternal MaterialsCo., Ltd.) and 50 parts by weight of polyestertetraacrylate (product name: Oligom...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com