Diagnosis of cancer by detecting auto-antibodies against EGF-receptor
a technology of egf receptor and auto-antibodies, which is applied in the field of epithelial cancer diagnosis, prognosis and treatment of ovarian cancer, can solve the problems of affecting the prognosis of cytoreductive surgery in patients with advanced epithelial ovarian cancer, affecting the prognosis of ovarian cancer, so as to achieve high egfr antibody levels
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0096]We measured the anti-EGFR autoantibody in serum samples using a sandwich ELISA kit (CellTrend GmbH Luckenwalde, Germany). The microtiter 96-well polystyrene plates were coated with membrane extracts of a cancer cell line “A431” human EGFR of SEQ ID NO:1. To maintain the conformational epitopes of the receptor, 1 mM calcium chloride was added to every buffer. Duplicate samples of a 1:100 serum dilution were incubated at 4° C. for 2 hours. After washing steps, plates were incubated for 60 minutes with a 1:20.000 dilution of horseradish-peroxidase—labeled goat anti-human IgG (Jackson, USA) used for detection. In order to obtain a standard curve, plates were incubated with test sera from an anti-EGFR autoantibody positive index patient. The ELISA was validated according to the FDA's “Guidance for industry: Bioanalytical method validation”.
[0097]To set a standard for the concentrations of the autoimmuno antibodies, a standard curve was generated In detail, a serum sample of a syste...
example 2
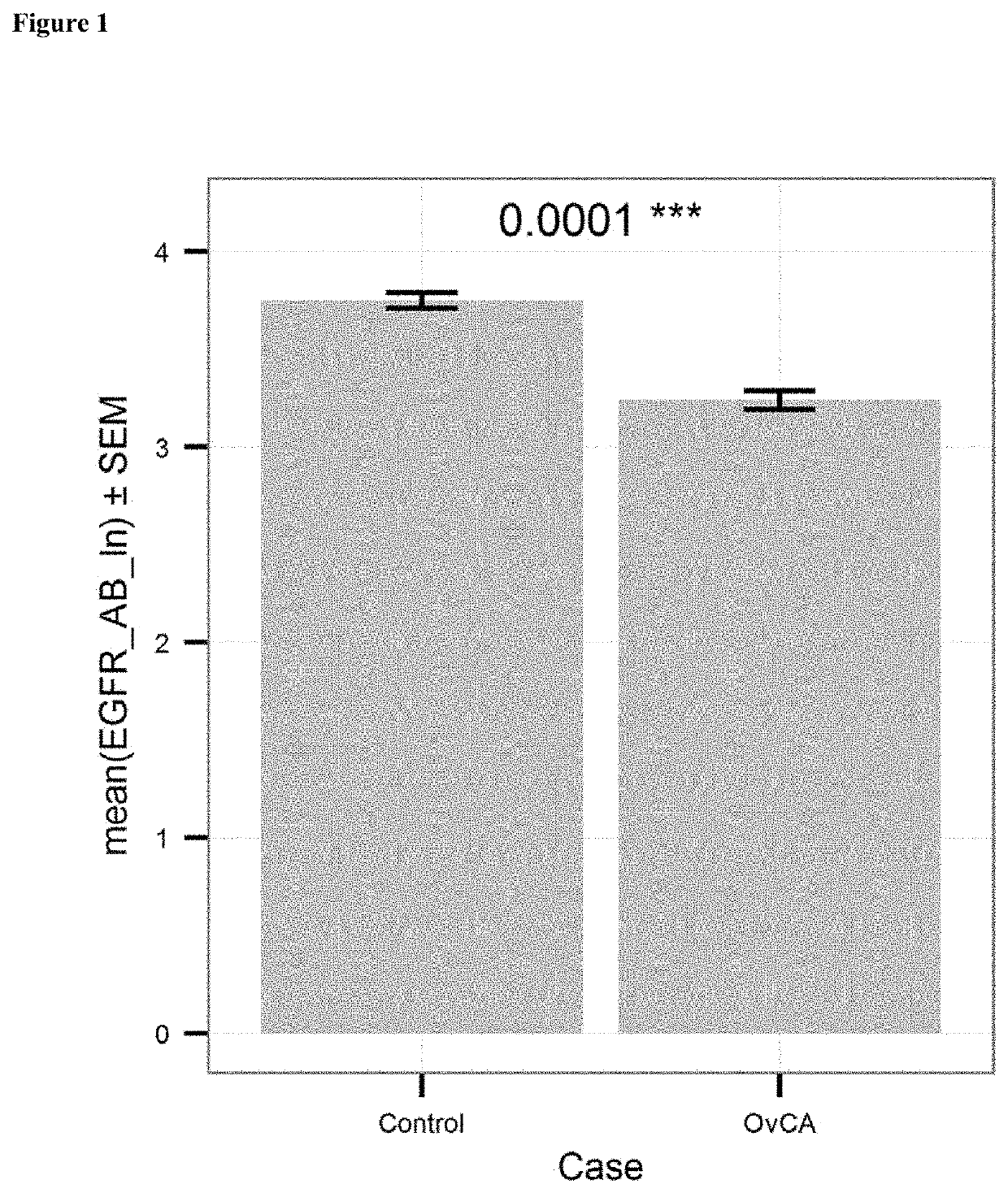
[0098]Anti-EGFR antibody levels in serum samples from 131 healthy donors (“control”) and 201 patients with ovarian cancer (“case”) were measured using the kit and method of Example 1. The levels were determined in units / mL. FIG. 1 shows the mean values of the natural logarithm of the EGFR antibody level for case and control subjects. Patient suffering from ovarian cancer had significantly lower levels (p≤0.0001) of anti-EGFR antibodies as compared to healthy controls.
example 3
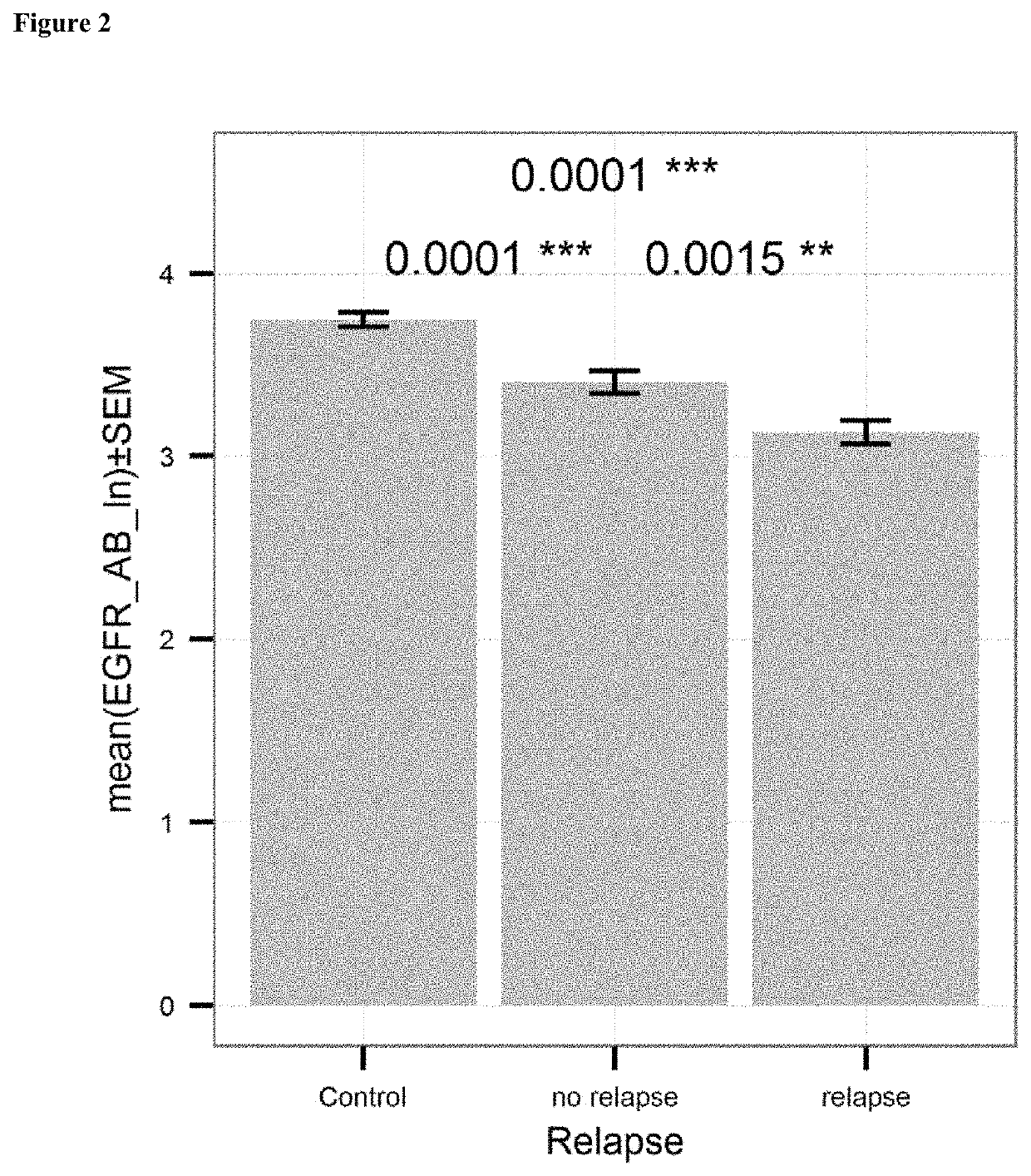
[0099]Levels of the EGFR-antibody were compared in patients showing relapse of ovarian cancer after therapy and patients showing no relapse. Treatment was surgical removal of the tumor and subsequent chemotherapy with cis platinum or carboplatinum conducted and monitored by physicians. Samples of patients were taken before treatment. Patients were categorized as “relapse” or “no relapse” according to the reoccurrence of cancer after a period of 24 months. Levels of EGFR-antibody were determined as outlined in Example 1. EGFR-AA levels are significantly higher in patients who had no relapse, compared to patients who had a relapse (p=0.0044). The determined levels were also compared to the control group of healthy subjects. These results are shown in FIG. 2.
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical density | aaaaa | aaaaa |
| fluorescent immunoassay | aaaaa | aaaaa |
| chemiluminescent assay | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com