Compositions and methods for treating heart failure in diabetic patients
a technology for diabetic patients and compositions, applied in the field of compositions and methods for treating heart failure in diabetic patients, can solve the problems of impaired calcium handling in diabetic patients, impaired renin-angiotensin system, and marked increase in the risk of coronary artery disease in dm
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
The Effect of Neucardin™ Administration by Different Routes on the Survival Rate of Rats with CHF
[0052]Introduction
[0053]In this study, we used a coronary artery ligation (CAL) model mimicking the myocardial infarction in the diabetic patients to investigate whether administration of Neucardin™ by IV drip using a by micro-injection pump or by subcutaneous (SC) bolus had any effects on survival rate and cardiac hemodynamics, 120 days after the initiation of administration of Neucardin™ 4 weeks after CAL. Echocardiography and cardiac remodeling were also used to determine cardiac function and recovery from CAL.
[0054]2. Methods:
[0055]2.1. Test Animals:
[0056]Strain, Origin: Wistar rats, Shanghai SLAC Laboratory Animal CO. LTD; Weight, 200±10 g, male;
[0057]2.2 Test Article:
[0058]2.2.1 Neucardin™
[0059]Identification: Recombinant human neuregulin-1 for injection (rhNRG-1, Neucardin™)
[0060]Lot Number: 200607009
[0061]Manufacturer: Zensun (Shanghai) Sci & Tech Co., Ltd
[0062]Dose form: Lyophil...
example 2
A Randomized, Double-Blinded, Multi-Center, Placebo Controlled Study to Evaluate the Efficacy and Safety of Recombinant Human Neuregulin 1 in Patients with Chronic Heart Failure Based on Standard Treatment
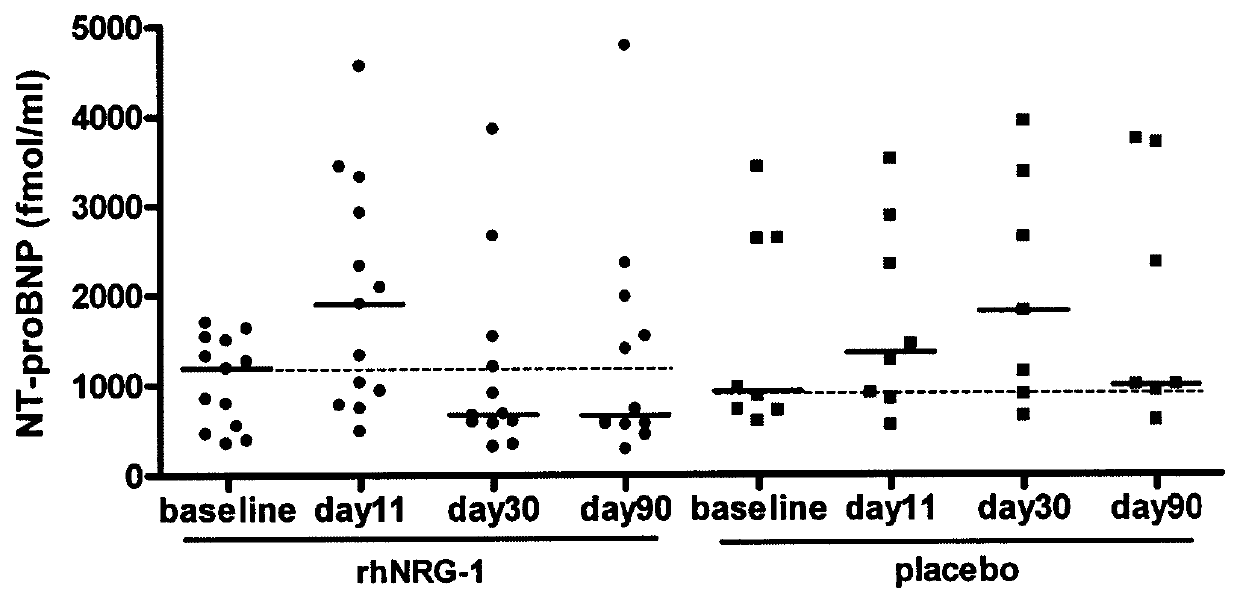
[0100]To evaluate the efficacy of recombinant human neuregulin-1 for injection on chronic heart failure including those diabetic patients with chronic heart failure, a phase II, double-blinded, multi-center, placebo controlled, standard treatment based study was carried out in multiple clinical centers in China. A total of 195 patients with NYHA Class II or III stable chronic heart failure including 21 diabetic patients were enrolled and randomized into three groups: placebo, or 0.6 μg / kg and 1.2 μg / kg of rhNRG-1. There were 13 patients with diabetic in Neucardin group (5 in 0.6 μg / kg / day arm and 8 in 1.2 μg / kg / day arm), and 9 patients in placebo group. There were no significant variations in demographics or background therapies among groups. According to the schedule, patients wer...
example 3
A Randomized, Double-Blinded, Multi-Center, Placebo Controlled Survival Study of Recombinant Human Neuregulin 1 in Patients with Chronic Heart Failure Based on Standard Treatment
[0114]To evaluate the efficacy of recombinant human neuregulin-1 for injection on chronic heart failure including those diabetic patients with chronic heart failure, a phase II, double-blinded, multi-center, placebo controlled, standard treatment based study was carried out in multiple clinical centers in China. A total of 351 patients with NYHA Class III or IV stable chronic heart failure were enrolled and randomized into placebo group or rhNRG-1 group (0.6 μg / kg). 68 of 351 patients were diabetic patients, with 35 cases in placebo group, and 33 cases in Neucardin™ group. There were no significant variations in demographics or background therapies among groups. According to the schedule, patients were administered with the drug for 10 consecutive days in the hospital, after finishing the day 11 follow up, t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| time | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com