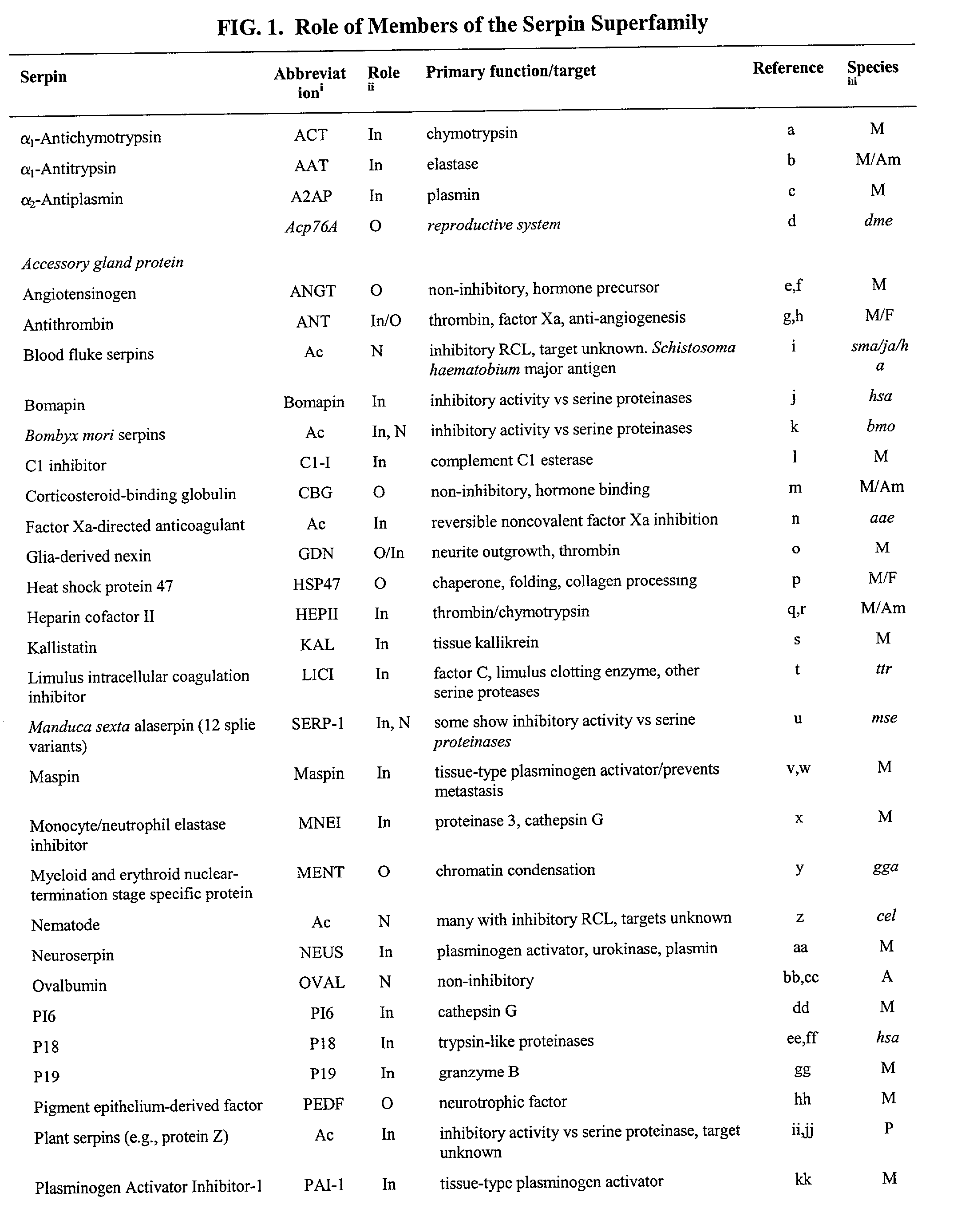
Serpin drugs for treatment of HIV infection and method of use thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
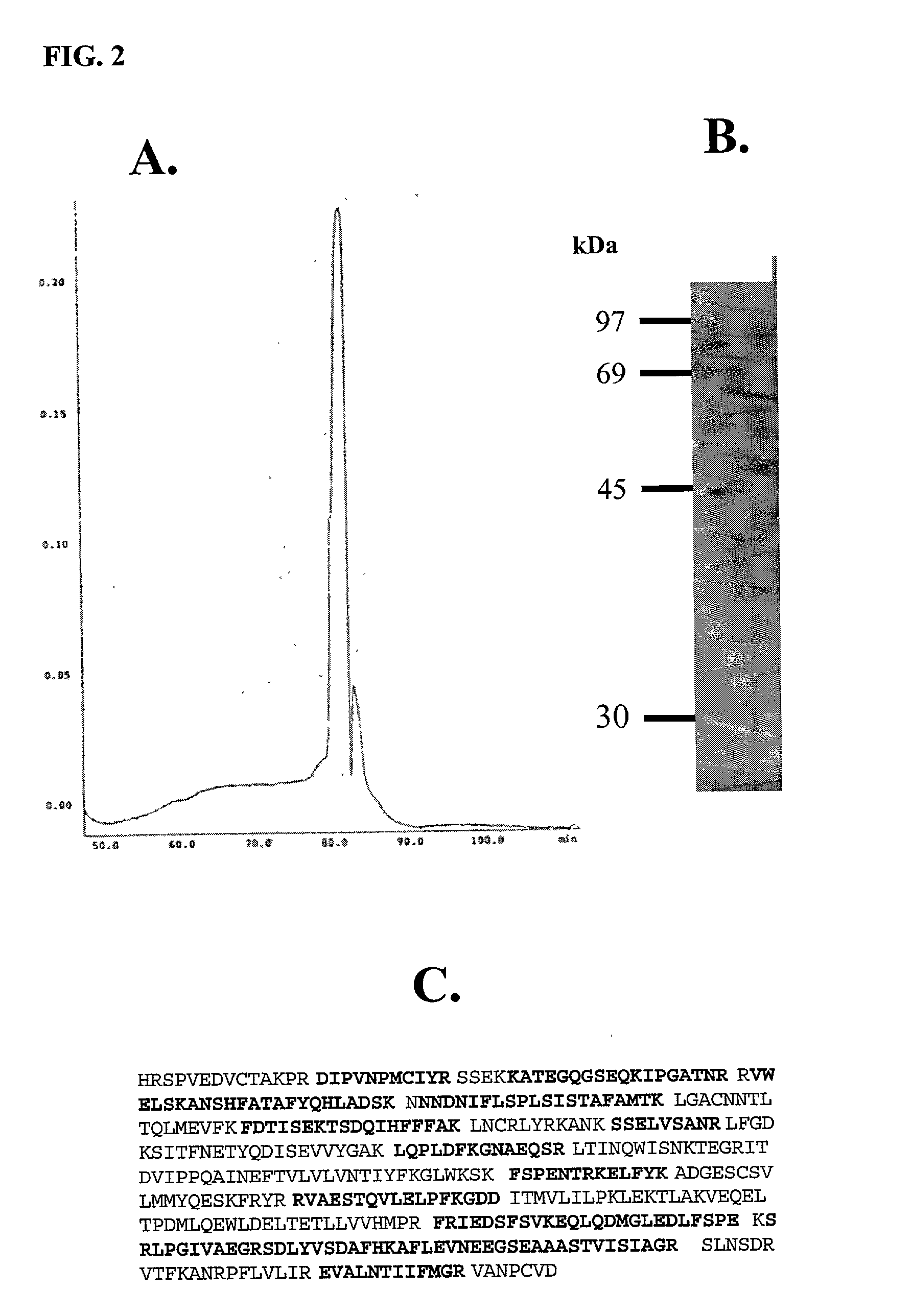
HPLC Purification of an HIV Inhibitory Factor from CD8.sup.+ T-Cells and its Identification as an Antithrombin III Using Nano-Electrospray Tandem Mass Spectrometry
[0134] To purify the ATIII-like HIV inhibitory factor, HIV-specific CTL or bulk CD8.sup.+ T-cells of long-term non-progressors were cultivated in vitro and stimulated with CD3 crosslinking (Geiben-Lynn et al., J. Virol., 75: 8306-16 (2001)) in either 10% heat-inactivated fetal bovine serum or 10% heat-inactivated human serum. After 4 h at 37.degree. C., media was collected, centrifuged, and applied to a heparin Sepharose column. The column was eluted with a continuous gradient to 1 M NaCl in phosphate-buffered saline (PBS, pH 7.4). Inhibitory fractions were pooled, and concentrated with a Centricon 50K centrifugal concentrator. The sample was applied to a Superdex 200 column. Fractions that inhibited were applied to a Vydac RP-4 HPLC column equilibrated with distilled water and 0.1% (v / v) trifluoroacetic acid (TFA) and tes...
example 2
Antiviral Activity of ATIII
[0137] 1. Comparative Evaluation of the Effect of Purified Bovine ATIII Forms on HIV, SIV, and SHIV Infectivity in vitro
[0138] To test the effect of ATIII on lentivirus infectivity (X4 HIV, R5 HIV, SIV.sub.239 or SHIV.sub.KU-1), cell lines (H9, PM1, SEM-174) were cultured in the presence or absence of the various forms of ATIII for up to nine days (Cf., FIGS. 3 and 4). Every three days (days 3, 6, and 9), 1 ml cell supernatant was removed from test wells and replaced with an equal volume of R20 culture medium containing either bovine ATIII or human ATIII. Control wells were similarly sampled, but received media without the ATIII supplement.
[0139] Both the test and the control wells were again sampled on the ninth day of culture and the concentration of the viral core protein p24 (gag) for the HIV (Alliance.RTM. HIV-1 p24 ELISA kit; NEN.RTM. Life Science, Boston, Mass., USA) or p27 antigen (SIV core antigen ELISA kit, Coulter, Miami, Fla.) was measured for ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Atomic weight | aaaaa | aaaaa |
| Composition | aaaaa | aaaaa |
| Volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com