Anti-Viral Azide Containing Compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Labeling HIV with Azide-Modified Biomolecules
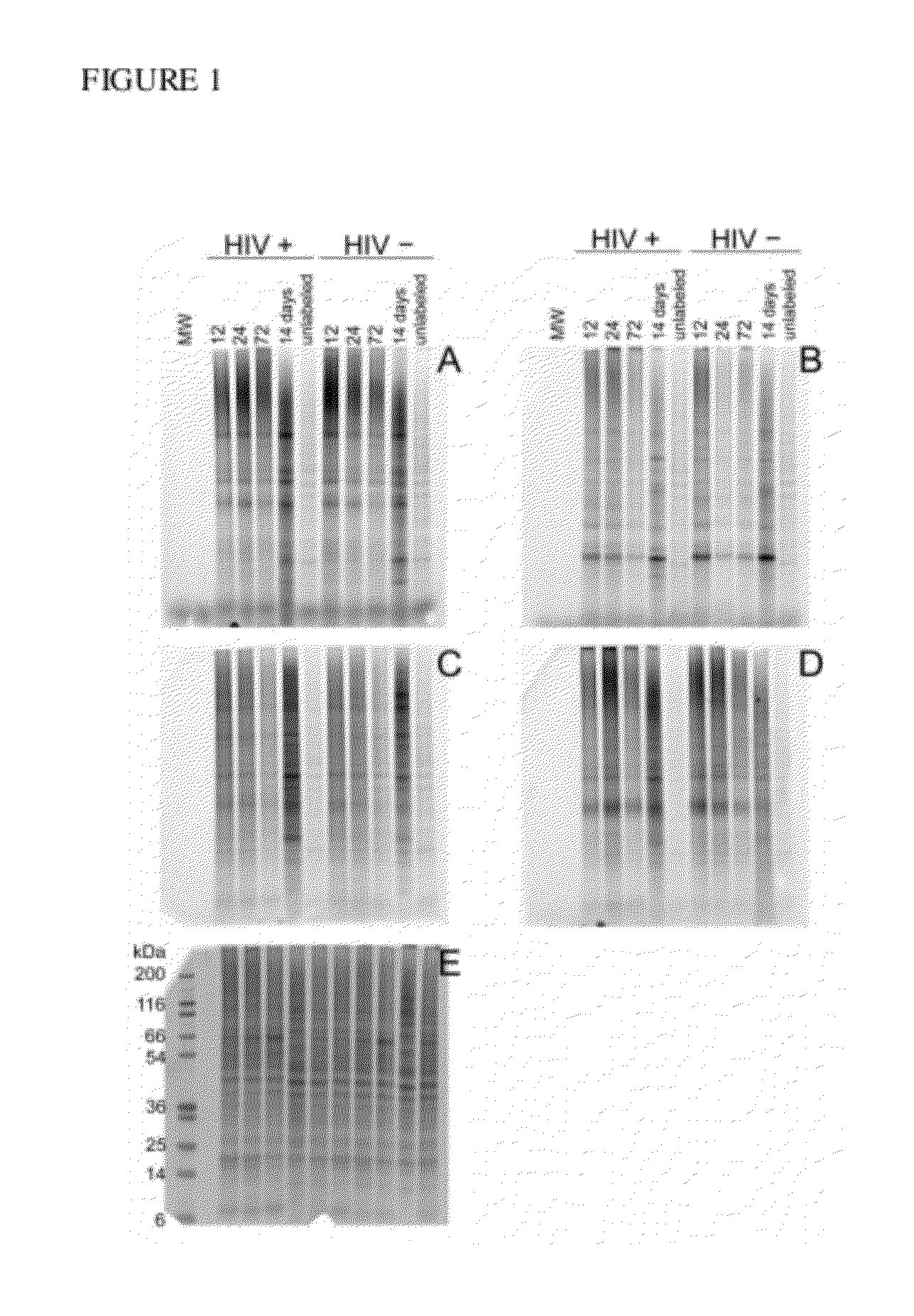
[0174]CEMx174 cells were transfected with HIVNL4-3 in a T-150 flask and the virus production was monitored by reverse transcriptase activity until peak virus production occurred (usually 7 to 9 days post transfection). Prior to transfection, the CEMx174 cells were spiked with the following azide-modified biomolecules: 15-azidopentadecanoic acid (50-100 μM), 12-azidododecanoic acid (50-100 μM), tetraacetylated N-azidoacetylgalactosamine (20-40 μM), and tetraacetylated N-azidoacetyl-D-mannosamine (20-40 μM).
[0175]Infected cells were harvested at 12, 24, 72 hours, and 14 days. Harvested cells were isolated and lysed. Cell lysates were then mixed with the CLICK-IT® detection reagent, tetramethylrhodamine (TAMRA) alkyne and the CLICK-IT® Protein Reaction Buffer Kit (Invitrogen, Carlsbad, Calif.). Cell lysate samples were run on a one-dimensional gel to monitor changes in the azide labeled proteins over time (FIG. 1).
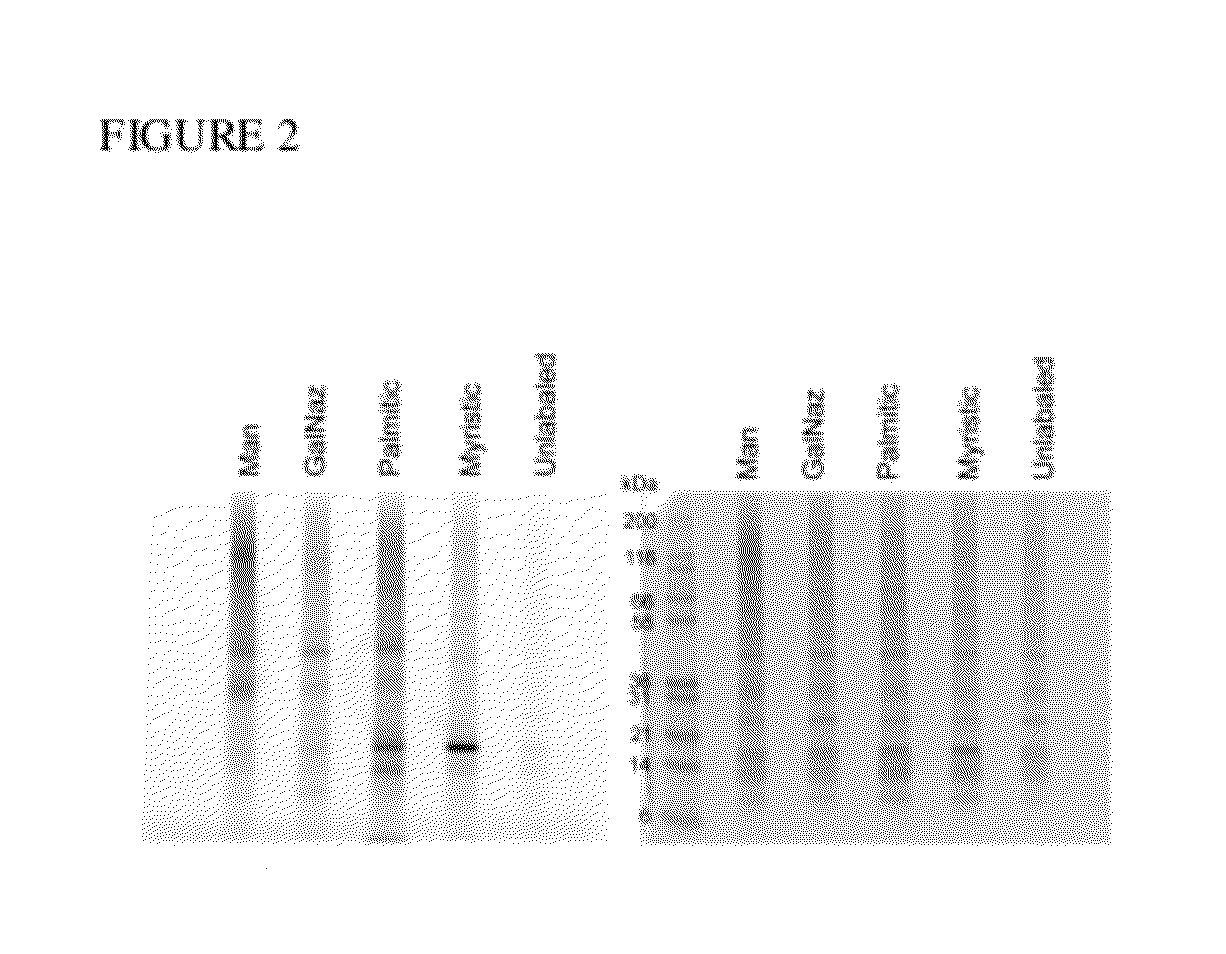
[0176]Labeled virus was als...
example 2
Inhibiting Infectivity of HIV
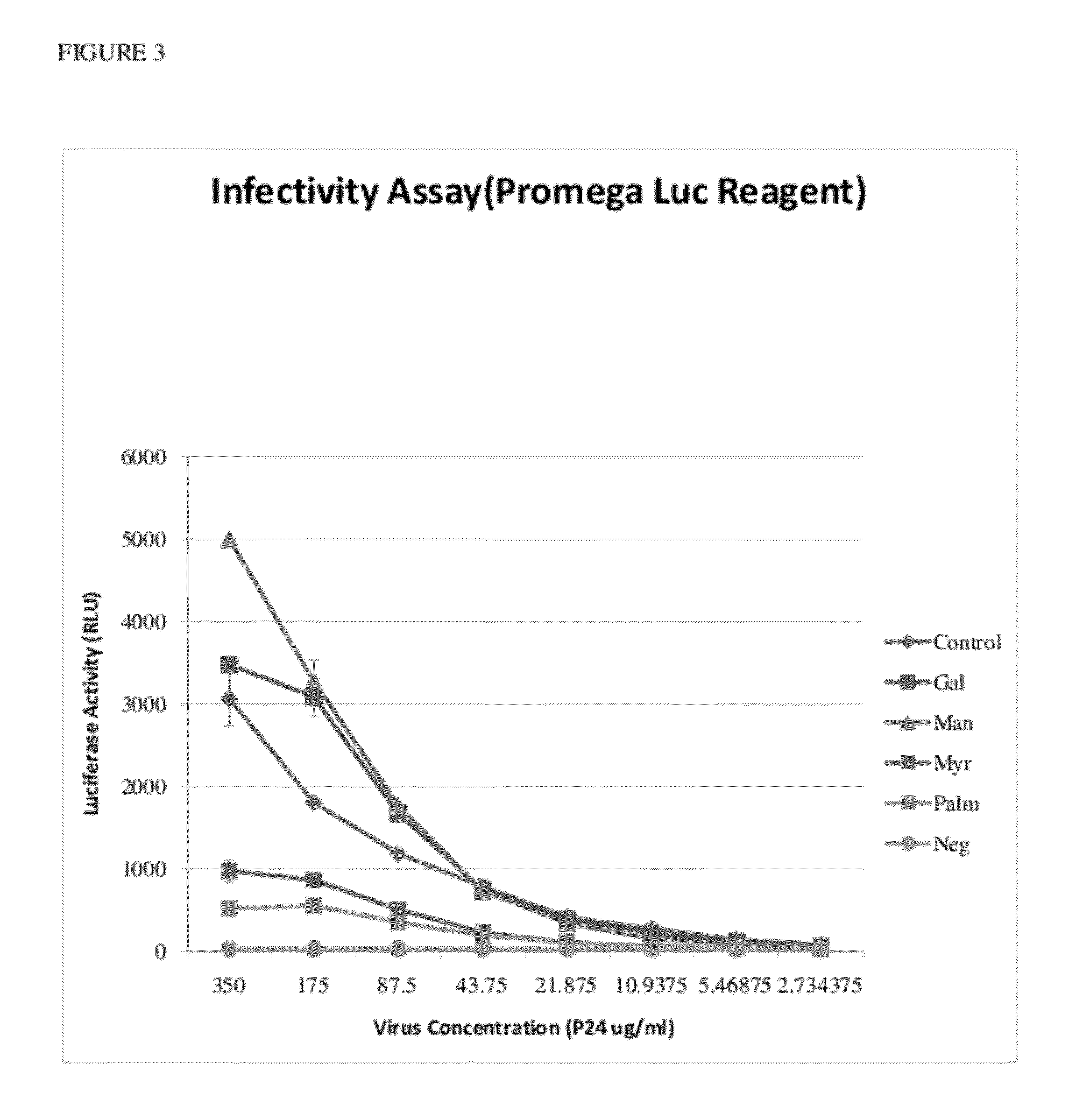
[0178]To examine the effect of the azide-modified biomolecules on the innate biology of the virus, the azide-labeled virus from the transfected cells was isolated and tested in cell infection studies. Unlabeled HIV, at a concentration 100 times less than the test samples, was used as a control. Viral loads were normalized to p24 abundance and virus incubated for 12 hours on a reporter cell line (TZM / BI). TZM / BI is a genetically engineered HeLa cell line that expresses CD4, CXCR4 and CCR5 and contains Tat-inducible luciferase and β-Gal reporter genes. Viral infectivity was determined by measuring cellular luciferase activities with two different luciferase reagents. The results using a single cycle replication system showed that virus labeled with the azide-modified biomolecules, particularly 12-azidododecanoic acid, and to a lesser extent, 15-azidopentadecanoic acid and tetraacetylated N-azidoacetylgalactosamine, had a profound impact on the infectivity ...
example 3
Toxicity Profile
[0179]Little to no toxicity has been observed using these azide-modified biomolecules in various eukaryotic cell lines, suggesting that these compounds have minimal toxicity profile and supporting their use in a therapeutic setting.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com