DDQ mediated one step dimerisation of beta-asarone or beta-asarone rich acorus calamus oil in the formation of novel neolignan
a technology of acorus calamus and beta-asarone, which is applied in the field of ddq mediated one step dimerisation of beta-asarone or beta-asarone rich acorus calamus oil in the formation of novel neolignan, can solve the problems of large reaction volume, difficult reproducibility, and maintain temperature, and achieve high purity of neolignan and side products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
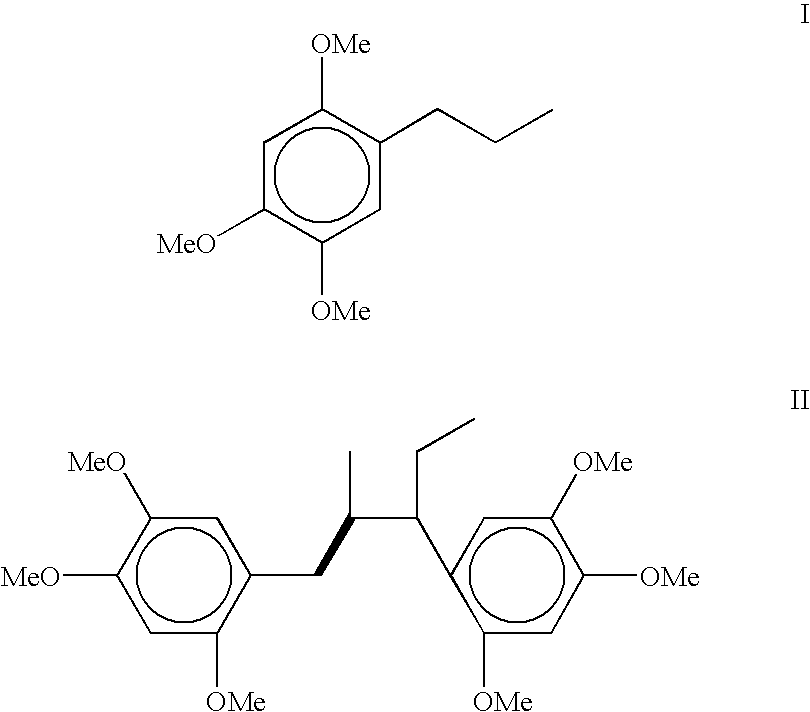
[0043] Preparation 2,4,5-trimethoxyphenylpropane (dihydro asarone): The starting material 2,4,5-trimethoxyphenylpropane is prepared by hydrogenation of either .beta.-asarone (isolated from Acorus calamus oil) or commercially available calamus oil rich in asarones (i.e. .beta. and / or .alpha.,.gamma.-asarone) content.
[0044] (a) Hydrogenation of .beta.-asarone into 2,4,5-trimethoxyphenylprop-ane (dihydro asarone): .beta.-asarone was isolated by loading the crude calamus oil (17.00 g) on silica gel column and then eluted the column with hexane to remove unwanted non-polar compounds. Subsequent elution with hexane-ethylacetate mixture with increasing proportion of ethylacetate upto 10% gave 13.94 g (82%, w / w) of pure liquid; R.sub.f 0.63 (hexane:toluene: ethylacetate=1:1:0.1); .sup.1H NMR (CDCl.sub.3, 300 MHz) .delta.6.84 (1H, s, H-6), 6.53 (1H, s, H-3), 6.50 (1H, dd, J=15.8 Hz and 1.5 Hz, H-1'), 5.78 (1H, dq, J=6.5 Hz and 15.8 Hz, H-2'), 3.88, 3.83 and 3.79 (s, 3H, each, 3-OCH.sub.3) an...
example ii
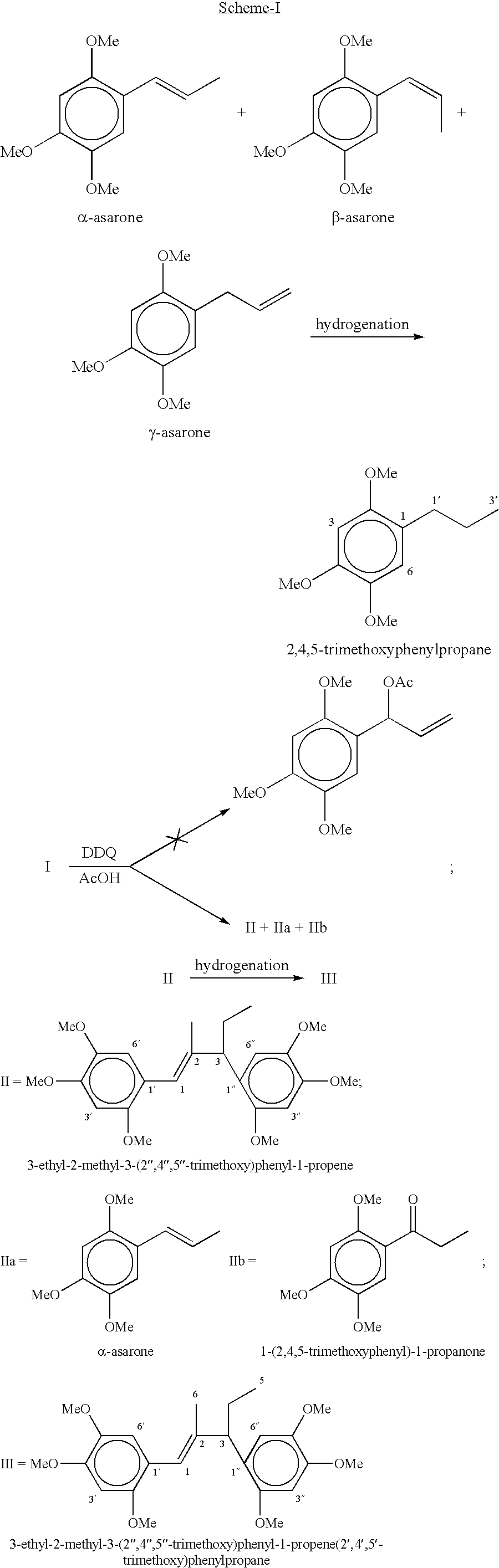
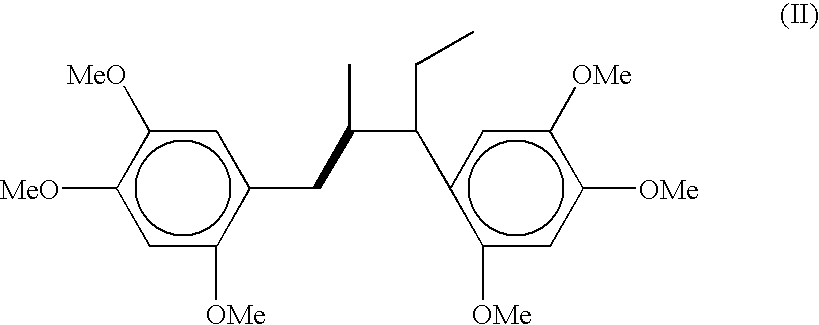
[0047] Preparation of 3-ethyl-2-methyl-3-(2",4",5"-trimethoxy)phenyl-1-(2'-,4',5'-trimethoxy)phenyl-1-propene: DDQ (6.13-7.97 g) was added over a period of 10-15 min to a ice cold and well stirred solution of 2,4,5-trimethoxyphenylpropane (5.67 g, 0.027 mol) in acetic acid (55 mL) and stirring was continued at room temperature for over night. The precipitated solid of DDQH.sub.2 was filtered and the filter cake washed twice with acetic acid. The combined acetic acid layer was evaporated and mixture was poured into water and extracted with dichloromethane (3.times.70 mL). The combined organic layer were washed with brine (3.times.15 mL), 10% sodium bicarbonate (2.times.10 mL), brine (3.times.15 mL) and dried over sodium sulphate. The residue obtained on evaporation of the solvents was chromatographed on silica gel using hexane-ethyl acetate mixture with increasing proportion of ethyl acetate upto 40% and the fractions having similar R.sub.f were mixed which after evaporation of solve...
example iii
[0052] Preparation of 3-ethyl-2-methyl-3-(2",4",5"-trimethoxy)phenyl-1-(2'-,4',5'-trimethoxy)phenylpropane: 0.20 mg of 5% Pd / C was added to a solution of 3-ethyl-2-methyl-3-(2",4",5"-trimethoxy)phenyl-1-(2',4',5'-tr-imethoxy)phenyl-1-propene (0.35 g, 0.84 mmole) in ethyl acetate (40 mL) and methanol (25 mL) and was shaken under atmosphere of hydrogen in paar reactor (5-20 psi) at room temperature till the disappearance of starting material. The catalyst was filtered and the solvent was removed under reduced pressure, which afforded a liquid. The liquid was purified on silica gel using above eluent system (hexane-ethyl acetate mixture) gave 3-ethyl-2-methyl-3-(2",4",5"-trimethoxy)phenyl-1-(2',4',5'-trimethoxy)phe-nylpropane (0.32 g) as a liquid in 91% yield; R.sub.f 0.47 (20% ethylacetate in hexane); .sup.1H NMR (CDCl.sub.3) .delta.6.77 (1H, s, H-3"), 6.68 (1H, d, H-6"), 6.54 (1H, d, H-6'), 6.51(1H, s, H-3'), 3.96 (6H,s, 2'-OCH.sub.3 and 2"-OCH.sub.3), 3.84 (6H, s, 4'-OCH.sub.3 and 4...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pressure | aaaaa | aaaaa |
| molar ratio | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com