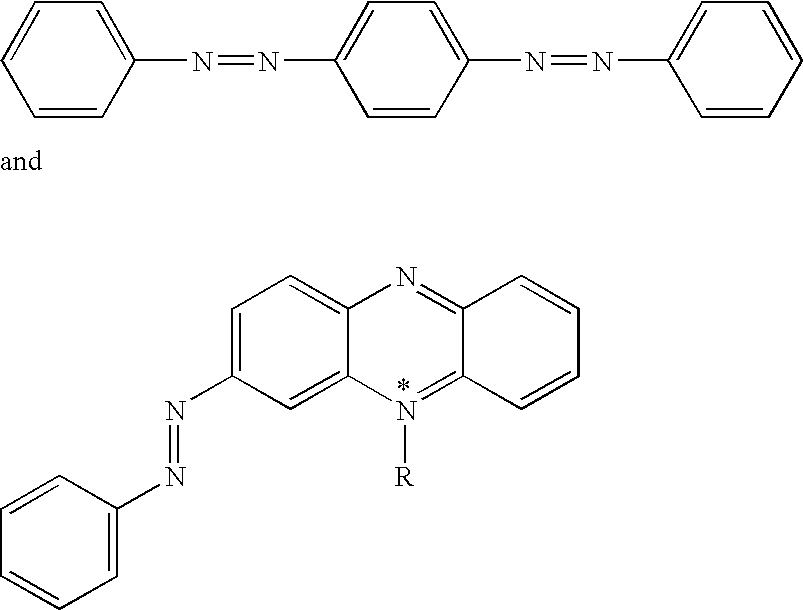
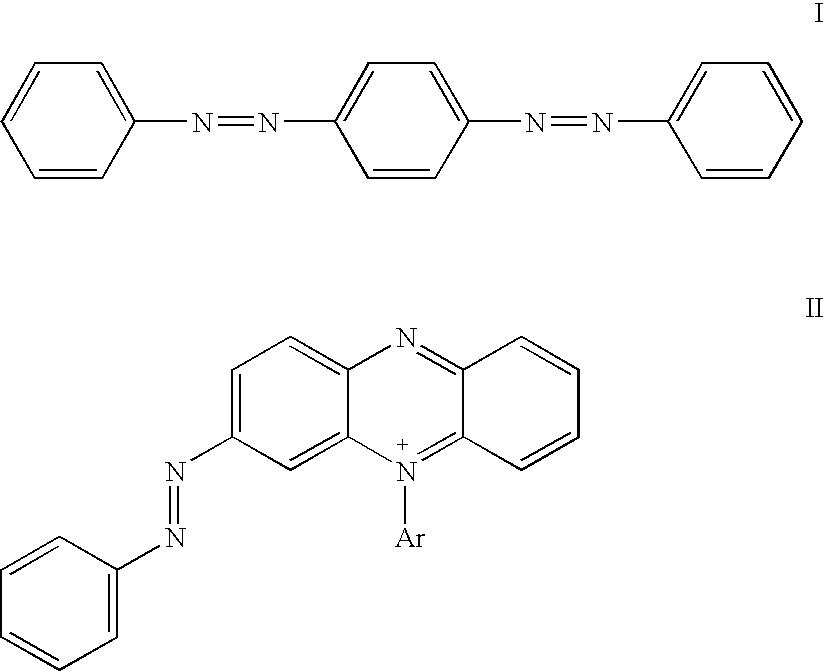
Non-fluorescent quencher compounds and biomolecular assays
a technology of non-fluorescence quencher compounds and biomolecular assays, applied in the field of molecular biology, can solve problems such as significant limitations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of Ethyl 4-[(2,5-dimethoxyphenyl)ethylamino]butanoate 4
[0146] 24
[0147] 2,5-Dimethoxyaniline 1 (7.7 gm, 0.050 moles) was dissolved in 40 ml tetrahydrofuran (THF) and chilled to 0.degree. C. under argon. Acetic anhydride (7.72 ml, 0.082 moles) was added. After stirring for 2 hours, thin-layer chromatography (TLC) indicated the reaction was complete. The mixture was evaporated under reduced pressure and kept under vacuum overnight.
[0148] Crude 2,5-dimethoxyacetanilide 2 was dissolved in 80 ml THF and stirred under argon. Borane-methylsulfide complex (62.5 ml, 2.0M in THF, 0.125 moles) was added by syringe over 10 minutes. After 10 minutes at ambient temperature, the mixture was heated to reflux for 3 hours. Analysis by TLC showed only a trace of 2. After cooling to room temperature, 42 ml water was cautiously added with stirring, followed by 4.5 ml conc. HCl. The reaction was heated for 1 hour. Next added sequentially with vigorous stirring were 125 ml water, 250 ml dichlorom...
example 2
Synthesis of Ethyl 4-(ethylphenylamino)butanoate 6
[0150] 25
[0151] N-ethylaniline 5 (2.0 gm, 0.016 moles), triethylamine (5.0 ml, 0.036 moles), and ethyl 4-bromobutyrate (3.45 gm, 0.018 moles) were heated at 110.degree. C. for 14 hours under argon. The mixture was cooled to room temperature, diluted with 50 ml ethyl acetate, washed with 50 ml water and 50 ml saturated NaCl, dried over MgSO.sub.4, filtered, and evaporated under reduced pressure to give crude ethyl 4-(ethylphenylamino)butanoate 6 (3.0 gm, 77% yield). .sup.1H NMR (CDCl.sub.3) .delta.7.2, 2H, m; 6.6, 3H, m; 4.15, 2H, q; 3.3, 4H, m; 2.38, 2H, t; 1.9, 2H, m; 1.25, 3H, t; 1.10, 3H, t.
example 3
Synthesis of Methyl 2-(ethylphenylamino)acetate 7
[0152] 26
[0153] N-Ethylaniline 5 (2 g, 16.52 mmol) was added to a stirred suspension of sodium hydride (436 mg, 18.17 mmol) in DME (20 ml) at ambient temperature under argon. After 45 minutes, bromomethyl acetate (3.02 g, 18.17 mmol) was added and the reaction mixture was stirred for 22 hr. DMF was removed under reduced pressure and the residue was treated with ethyl acetate (60 ml) and water (50 ml). The organic extract was washed with saturated brine (50 ml), dried (Na.sub.2SO.sub.4), filtered and evaporated to give crude Methyl 2-(ethylphenylamino) acetate 7 as liquid (3.06 g, 96%).
PUM
| Property | Measurement | Unit |
|---|---|---|
| absorption maximum at wavelengths | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| Tm | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com