Cross-linked high amylose starch for use in controlled-release pharmaceutical formulations and processes for its manufacture
a technology of high amylose starch and pharmaceutical formulation, which is applied in the direction of drug composition, antibacterial agents, nervous disorders, etc., can solve the problem of inability to have controlled release properties of materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0092] Preparation of Controlled Release Excipient
[0093] A. Preparation of Cross-Linked High Amylose Starch
[0094] High amylose starch (30.0 kg) containing about 70% w / w of amylose (CI AmyloGel 03003) is placed in a reactor. To this reactor is added water (55.0 1) containing sodium hydroxide (30.0 g) and sodium sulfate (2.40 kg). The resulting slurry is heated to a temperature of 30.degree. C. Phosphorus oxychloride (22.5 g) is added to the reaction mixture which is reacted for one hour.
[0095] B. Preparation of Hydroxypropylated Cross-Linked High Amylose Starch
[0096] The crude reaction mixture from Part A is transferred into a hydroxypropylation reactor. The reaction mixture is heated to 40.degree. C. over 30 minutes and the reaction is purged with nitrogen. After a full purge, propylene oxide (1.80 kg) is added. The reaction mixture is kept at 40.degree. C. for 20 hours. The reaction mixture is neutralized with 0.1N H2SO4 (1:2 v / v) to a pH of 5.5. The starch slurry is washed with a ...
example 2
[0102] Preparation of Controlled Release Excipient
[0103] A. Preparation of Cross-Linked High Amylose Starch
[0104] High amylose starch (30.0 kg) containing about 70% w / w of amylose (CI AmyloGel 03003) is placed in a reactor. To this reactor is added water (55.0 1) containing sodium hydroxide (30.0 g) and sodium sulfate (2.40 kg). The resulting slurry is heated to a temperature of 30.degree. C. Sodium trimetaphosphate (45 g) is added to the reaction mixture which is reacted for one hour.
[0105] B. Preparation of Hydroxypropylated Cross-Linked High Amylose Starch
[0106] The crude reaction mixture from Part A is transferred into a hydroxypropylation reactor. The reaction mixture is heated to 40.degree. C. over 30 minutes and the reaction is purged with nitrogen. After a full purge, propylene oxide (1.80 kg) is added. The reaction mixture is kept at 40.degree. C. for 20 hours. The reaction mixture is neutralized with 0.1N H2SO4 (1:2 v / v) to a pH of 5.5. The starch slurry is washed with a b...
example 3
[0111] Preparation of Controlled Released Tramadol HCl 100 mg Tablets
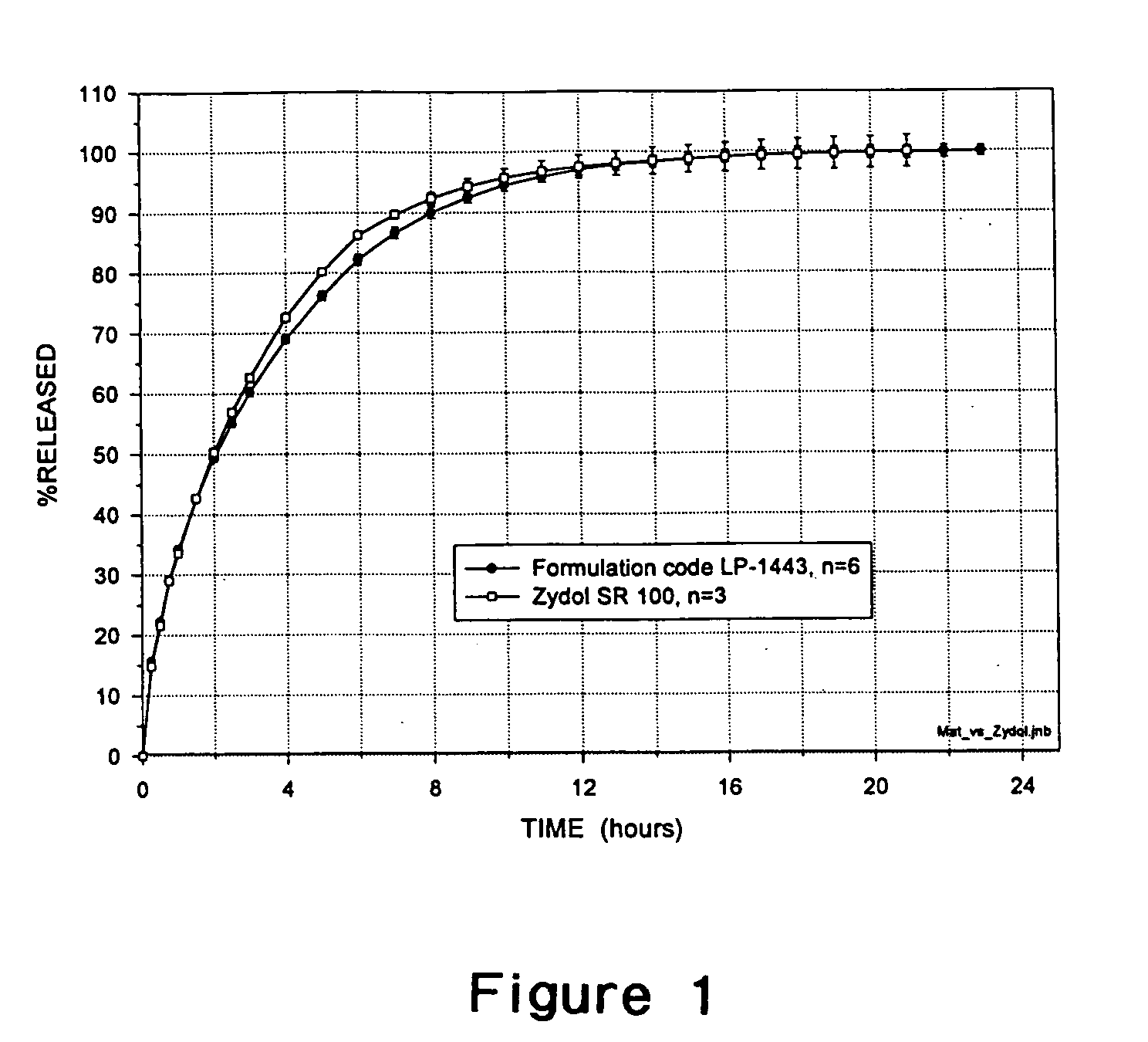
[0112] Tramadol HCl 100 mg tablets were prepared in a matrix dosage form (Formulation LP- 1443) with cross-linked high amylose starch prepared as described in Example 1. The components of Formulation LP-1443 are listed in Table 1. The Formulation LP-1443 tablets have a diameter of 9.53 mm. The shape of an LP-1443 tablet is round and biconvex. For comparison, Tramal Long 100.RTM. (manufactured by Grunenthal, Germany) was used. Tramal Long 100.RTM. contains 100 mg of Tramadol HCl and are in a matrix dosage form with a diameter of 10.15 mm. The shape of Tramal Long 100.RTM. is round and biconvex.
4TABLE 1 Description of formulation LP-1443 Ingredients Quantity per tablet (mg) % (w / w) Tramadol HCl 100 30.77 cross-linked high amylose 188.6 59.03 starch Xanthan gum 32.5 9 Talc (USP) 3.25 1 SiO.sub.2 0.65 0.2 TOTAL 325 100
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com