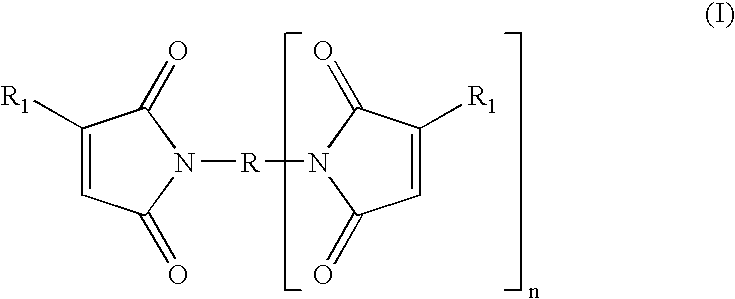
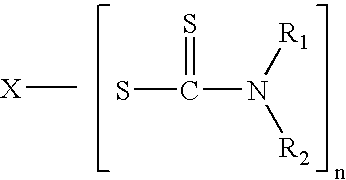
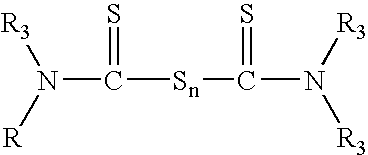
Tack free surface cures of polymers by organic peroxides in the presence of air
a technology of organic peroxide and polymer, which is applied in the field of organic peroxide free surface cure of polymers in the presence of air, can solve the problems of laborious surface coating technique, adverse effects of elemental sulfur on the final physical properties of cured elastomers, etc., and achieves the reduction or no surface tack, the effect of increasing the efficiency of free radical cure and reducing the efficiency of free radical initiators
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Evaluation of Prior Art Compositions for Crosslinking EPM in the Presence of Air and their Effect on Surface Tack and Level of Crosslinking
[0219] In the example, various known co-curing agents, e.g., unsaturated monomeric crosslinking coagents, elemental sulfur, or sulfur donor compounds, were evaluated in a cure with an organic peroxide. The elastomer used in this example was an ethylene propylene copolymer (EPM) marketed by Exxon, VISTALON.RTM. 504. Prior art (U.S. Pat. No. 4,983,685) claims that use of high levels (2.5 to 20 parts) of specific sulfur containing compounds with or without optional unsaturated monomers can be used to crosslink elastomers with peroxide in the presence of air and produce a non-tacky surface. Other references employ sulfur. In this example, low levels of sulfur or sulfur donor compounds were evaluated alone or with some monomeric coagents. The data in Table 1 shows these formulations produced a sticky surface, but also provided a crosslinked product wi...
example 2
Compounding of Prior Art Peroxide Formulations Versus Novel Peroxide Formulations for Crosslinking fully Saturated (no Double Bonds within the Polymer Chains) Elastomers
[0225] The novel peroxide formulation provides good physical properties associated with a conventional peroxide cure, plus an unexpected non-tacky surface when crosslinking in the presence of air.
[0226] In this example, 40% assay dicumyl peroxide dispersed on clay, was used to crosslink EPM (polyethylene propylene copolymer) VISTALON.RTM. 707 (Exxon). Table 2, run #1, dicumyl peroxide blended with SR 206 (ethylene glycol dimethacrylate) was evaluated for crosslinking in the MDR, % compression set and in hot air cure for surface tack. Note: prior art (U.S. Pat. No. 4,983,685) used SR 206 as an optional monomeric coagent in their examples. This peroxide and coagent blend produced a good cure (M.sub.H) and % compression set, with a very sticky surface upon hot air cure at 400.degree. F. Addition of Sulfads.RTM. (98% dip...
example 3
Novel Peroxide-Additive Compositions Produce Solid or Foamed Crosslinked Elastomer in Hot Air, with Outstanding Physical Properties, e.g. Percent Compression set, while also Producing a non Tacky Surface when Curing in the Presence of Air
[0234] The elastomer used in this example was Uniroyal.RTM. X3378 EPDM containing 4% dicyclopentadiene as the tenmonomer. Peroximon DC 40KEP (40% dicumyl peroxide on clay) was used as the peroxide crosslinking agent. In this example the levels of Sulfads.RTM. 98% dipentamethylene thiuram tetra sulfide), VULTAC.RTM. 5 (75% alkyl phenol disulfide polymer) and Durax.RTM. (98% N-cyclohexyl-2-benzothiazolesulfenamnide) were varied, keeping the peroxide and HVA-2 coagent constant (Table 3, runs #2 to #6).
[0235] Note: adding 0.5 parts (phr) Sulfads is equivalent to 0.49 parts of dipentamethylene thiuram tetra sulfide, per 100 parts of rubber. Referring to runs #2, #3 and #4, unexpectedly, excellent compression set and a tack free surface were found when us...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com