Ion channel modulating compounds and uses thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
(±)-TRANS-[2-(4-MORPHOLINYL)-1-(2-NAPHTHENETHOXY)]CYCLOHEXANE MONOHYDROCHLORIDE
COMPOUND #1
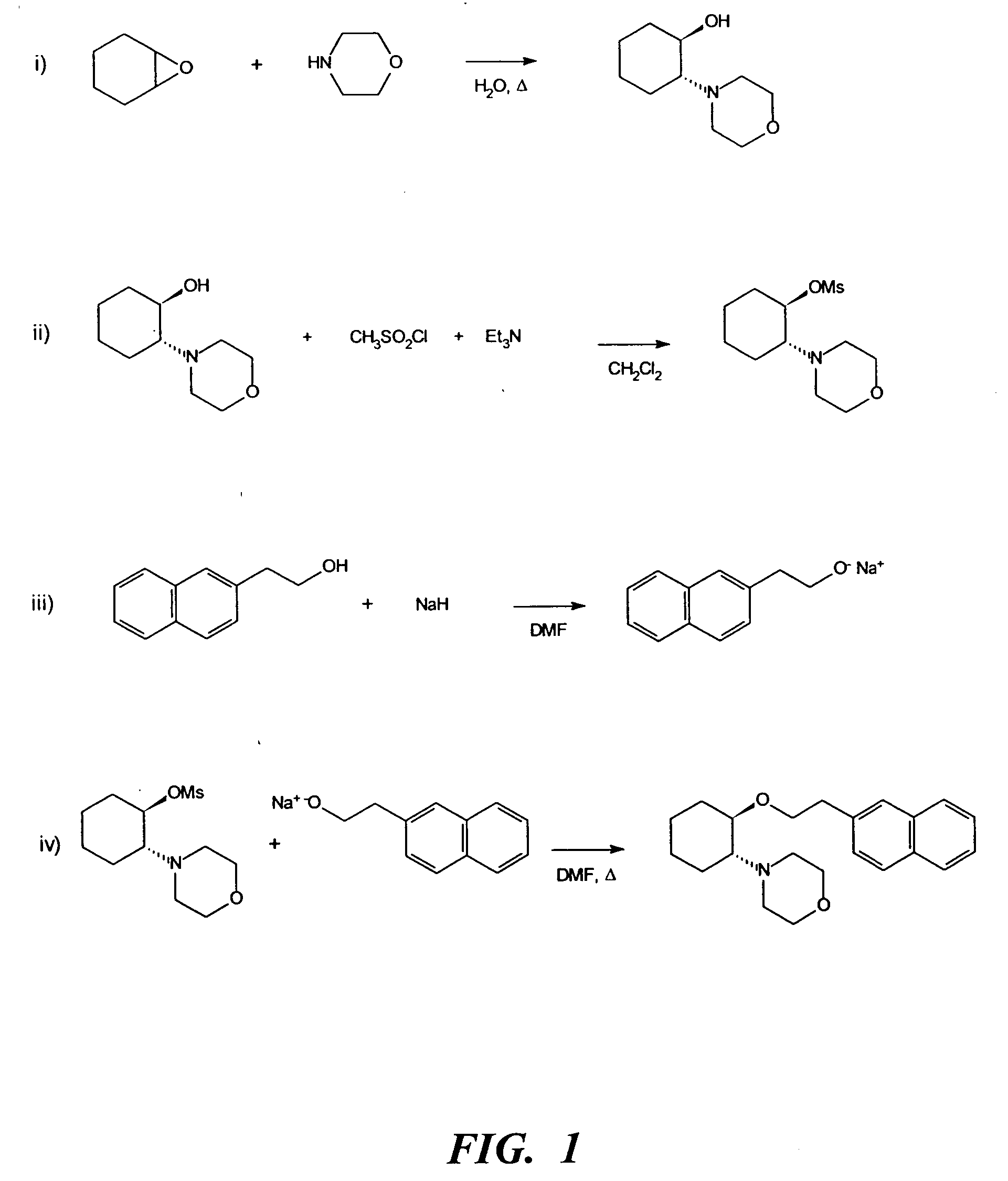
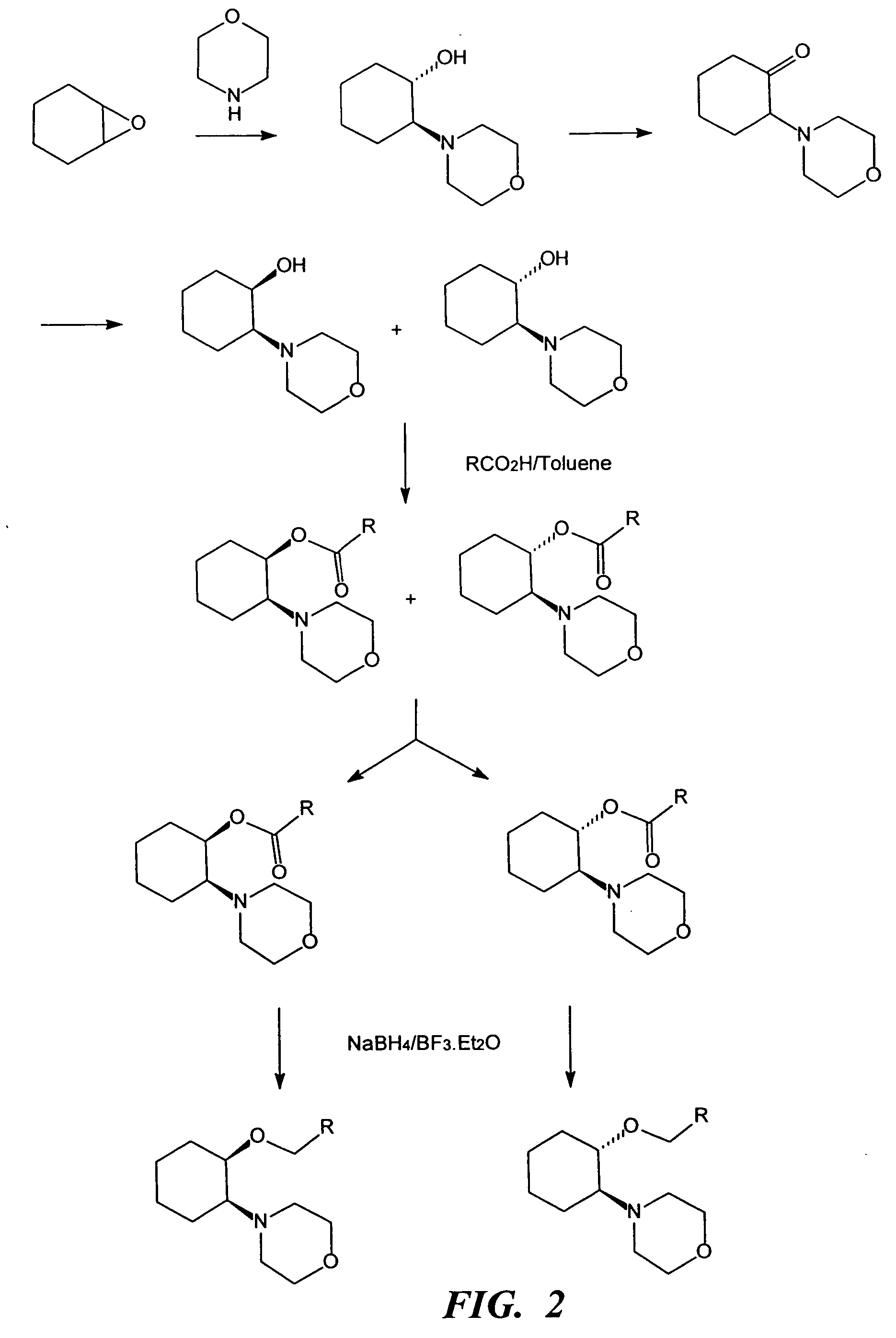
(i) Morpholine (5 mL, 57 mmol), cyclohexene oxide (5.8 mL, 57 mmol) and water (3 mL) were refluxed for 1.5 h. GC analysis showed the reaction to be complete. The cooled mixture was partitioned between saturated NaOH solution (50 mL) and ether (75 mL). The aqueous layer was backwashed with ether (30 mL) and the combined ether layers were dried over sodium sulfate. The ether was removed in vacuo to leave a yellow oil (9.83 g). The crude product, (±)-trans-[2-(4-morpholinyl)]cyclohexanol, was purified by vacuum distillation (b.p. 75-80° C. at full vacuum) to give a clear liquid (8.7 g). Yield 82.5%.
(ii) To a chilled (0° C.) solution of (±)-trans-[2-(4-morpholinyl)]cyclohexanol (6.0 g, 32.4 mmol) and triethylamine (6.8 mL, 48 mmol) in dichloromethane (100 mL) was added via cannula a solution of methanesulfonyl chloride (3.10 mL, 40 mmol) in dichloromethane (50 mL). The addition was completed i...
example 4
(±)-TRANS-[2-(4-MORPHOLINYL)-1-[2-(2-NAPHTHOXY)ETHOXY)]CYCLOHEXANE MONOHYDROCHLORIDE
COMPOUND #4
(i) The starting trans-aminocyclohexanol is prepared according to example 1.
(ii) To a chilled (0° C.) solution of (±)-trans-[2-(4-morpholinyl)]cyclohexanol (3.0 g, 16.2 mmol) and triethylamine (3.4 mL, 24 mmol) in dichloromethane (50 mL) was added via cannula a solution of methanesulfonyl chloride (1.55 mL, 20.0 mmol) in dichloromethane (50 mL). The addition was completed in 10 min., the reaction mixture was stirred for another hour at 0° C. and then at room temperature for 4 hours. The dichloromethane mixture was washed with water (2×50 mL) and the combined aqueous washings back extracted with dichloromethane (50 in 10 min., the reaction mixture was stirred for another hour at 0° C. and then at room temperature for 4 hours. The dichloromethane mixture was washed with water (2×50 mL) and the combined aqueous washings back extracted with dichloromethane (50 mL). The combined organic la...
example 5
(±)-TRANS-[2-(4-MORPHOLINYL)-1-[2-(4-BROMOPHENOXY)ETHOXY)]]CYCLOHEXANE MONOHYDROCHLORIDE
COMPOUND #5
(i) The starting trans-aminocyclohexanol is prepared according to example 1.
(ii) To a chilled (0° C.) solution of (±)-trans-[2-(4-morpholinyl)]cyclohexanol (3.0 g, 16.2 mmol) and triethylamine (3.4 mL, 24 mmol) in dichloromethane (50 mL) was added via cannula a solution of methanesulfonyl chloride (1.55 mL, 20.0 mmol) in dichloromethane (50 mL). The addition was completed in 10 min., the reaction mixture was stirred for another hour at 0° C. and then at room temperature for 4 hours. The dichloromethane mixture was washed with water (2×50 mL) and the combined aqueous washings back extracted with dichloromethane (50 mL). The combined organic layers were dried over sodium sulfate and concentrated in vacuo to provide 3.95 g (92% yield) of the crude mesylate.
(iii) To sodium hydride, 80% oil dispersion, previously washed with hexanes (3×10 mL), (0.63 g, 26 mmol) in dry dimethylformami...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Electrical conductance | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com