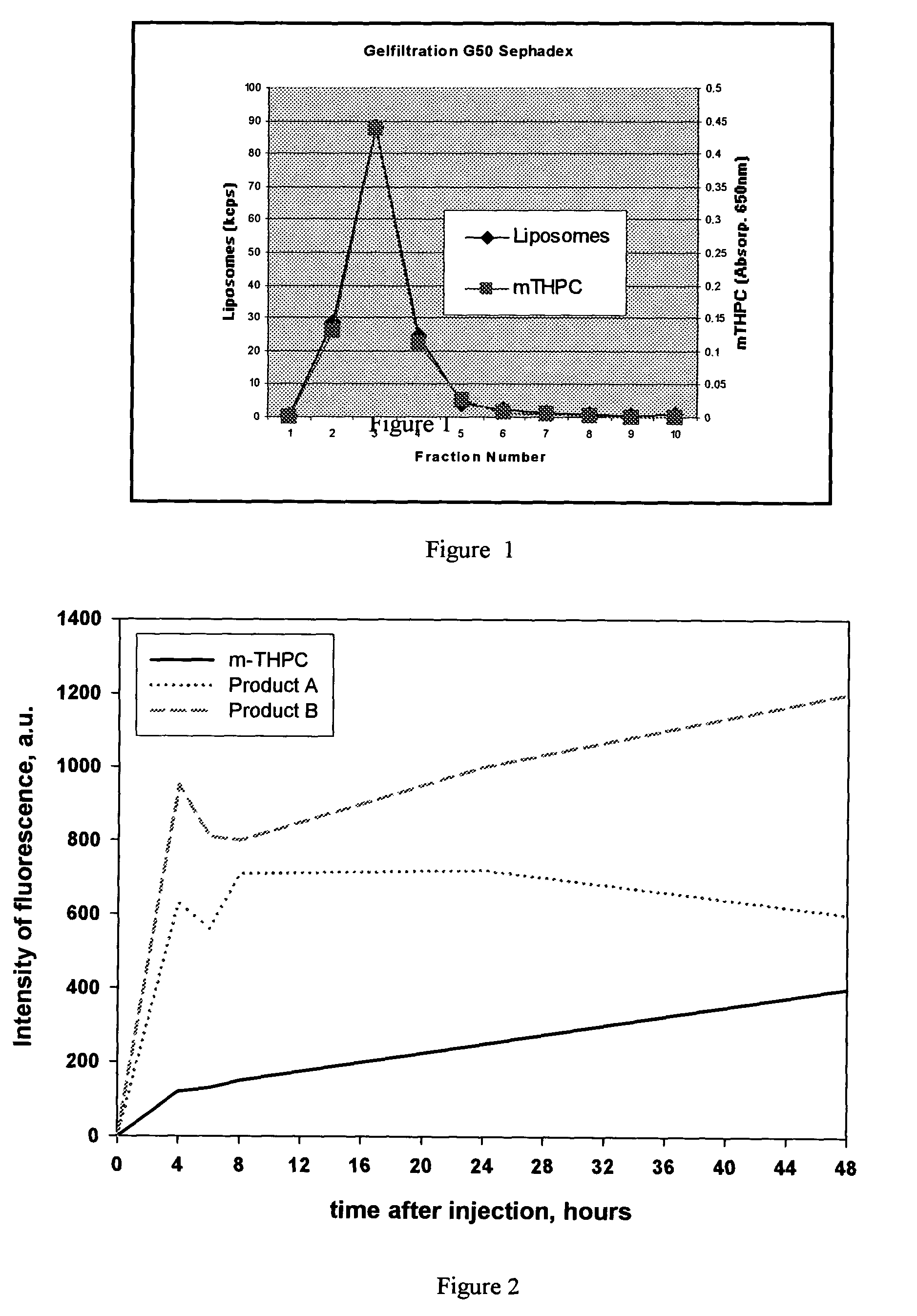
Non-polar photosensitizer formulations for photodynamic therapy
a non-polar, photodynamic therapy technology, applied in the direction of dermatological disorders, sexual disorders, drug compositions, etc., can solve the problems of lack of specificity, limited liposomes, patient gaining a new set of maladies from the therapy, etc., to improve the transport of non-polar photosensitizers, improve pharmacokinetic properties, and preserve the structure and size of liposomes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Liposomes containing m-THPC
mTHPC (Temoporfin) was synthesized as described in U.S. Pat. Nos. 4,992,257 and 5,162,519, incorporated herein by reference.
Liposomes were prepared according to the following general procedure:
Non-polar photosensitizer, ascorbic palmitate and the phospholipids are dissolved in chloroform / methanol. The solution is then dried under vacuum using a rotary evaporator until the chloroform / methanol mixture is not detectable by gas chromatography anymore. Water for injection is added to rehydrate the lipid film at a temperature of 50° C. for at least 2 hours. The mixture is then passed through a homogenizer filter system using a final pore size of 100 nanometer. Optionally, the rehydration water is supplemented with monosaccharides or polyalcohols. The filtrate is collected, filled into vials and optionally freeze dried. The freeze dried composition is reconstituted with water for injection prior to administration.
Using the foregoing procedu...
example 1a
IngredientAmount % w / vmTHPC 0.05 to 0.15Dipalmitoyl Phosphatidyl Choline 0.5 to 2.0Dipalmitoyl Phosphatidyl Glycerol 0.05 to 0.2pegylated Distearoyl Phosphatidyl 0.05 to 0.2EthanolamineAscorbic Palmitate0.002 to 0.004Water for Injectionas required to achievedesired concentrations above
example 1b
IngredientAmount % w / vmTHPC 0.05 to 0.15Dipalmitoyl Phosphatidyl Choline 0.5 to 2.0Dipalmitoyl Phosphatidyl Glycerol 0.05 to 0.2Ascorbic Palmitate0.002 to 0.004Water for Injectionas required to achievedesired concentrations above
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com