Electrode for nonaqueous electrolyte battery
a nonaqueous electrolyte, electrolyte technology, applied in the direction of liquid surface applicators, cell components, coatings, etc., can solve the problems of reduced life, difficult to stably store electric energy, and low safety of secondary batteries, etc., to achieve excellent safety, high rate charge-discharge properties, and excellent safety.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
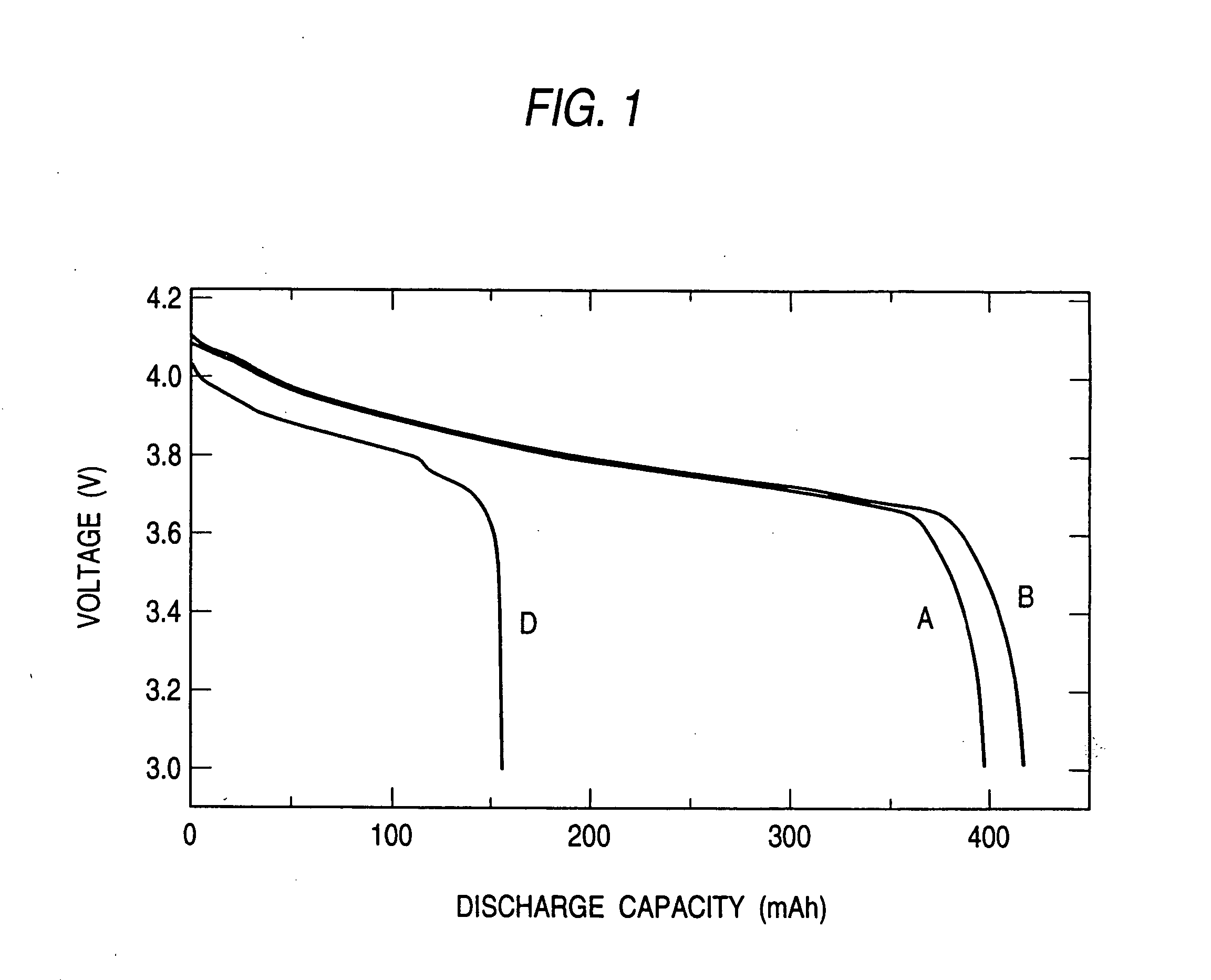
Image
Examples
Embodiment Construction
In the conventional liquid electrolyte lithium-based battery, the particulate active material is covered by an organic electrolyte solution and comprises the organic electrolyte solution in the pores. Thus, the particulate active material comes in contact with a large amount of the organic electrolyte solution. Since the organic electrolyte solution is combustible and has a higher chemical reactivity than an aqueous solution, the chemical reaction of the active material with the electrolyte solution can easily proceed when the inner temperature of the battery rises due to the shortcircuiting of the battery, external heating, etc. If this reaction is an exothermic reaction, it proceeds explosively, possibly causing the battery to be ignited and detonated. Further, since a lithium-based battery exhibits a higher battery voltage than an aqueous solution-based battery, it undergoes decomposition of the electrolyte solution due to oxidation or reduction while being stored charged when t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| electromotive force | aaaaa | aaaaa |
| electromotive force | aaaaa | aaaaa |
| electromotive force | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com