Intradermal delivery of substances
a technology of intradermal delivery and substance, which is applied in the direction of drug composition, peptide/protein ingredient, metabolic disorder, etc., can solve the problems of insufficient reproducibility of the transdermal delivery system, variable clinical results, and maintaining the placement of the needle in the intradermal space, so as to improve the clinical utility of id delivery, prevent the leakage of substances out of the skin, and improve the absorption within the intradermal space
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
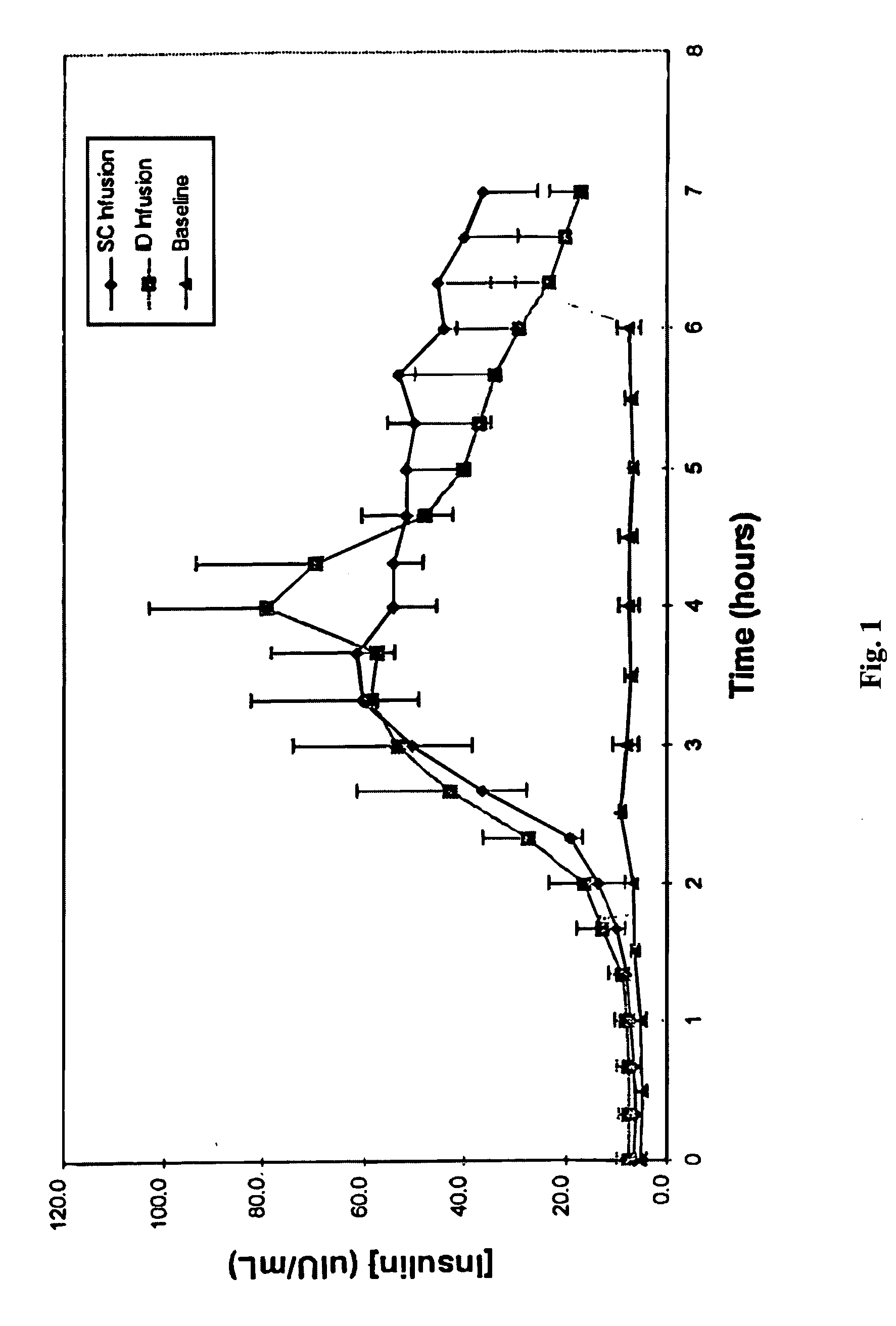
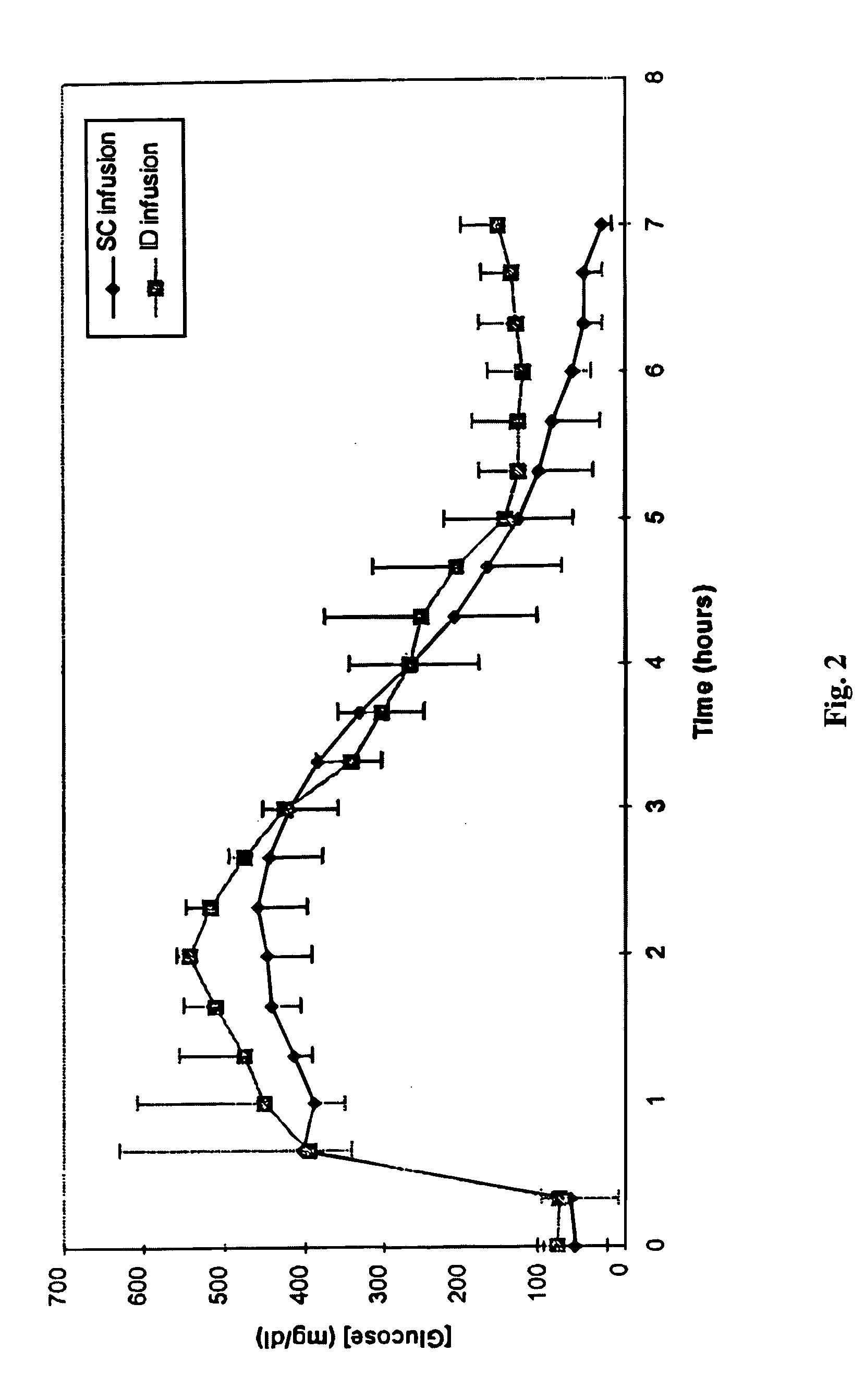
[0017] ID infusion of insulin was demonstrated using a stainless steel 30 gauge needle bent at the tip at a 90° angle such that the available length for skin penetration was 1-2 mm. The needle outlet (the tip of the needle) was at a depth of 1.7-2.0 mm in the skin when the needle was inserted and the total exposed height of the needle outlet was 1.0-1.2 mm. The needle was constructed in a delivery device similar to that described in U.S. Pat. No. 5,957,895, with infusion pressure on the insulin reservoir provided by a plastic Belleville spring and gravimetrically measured flow rates of 9 U / hr (90 mL / hr). The corresponding flow rates for SC control infusions were set using MiniMed 507 insulin infusion pumps and Disetronic SC catheter sets. Basal insulin secretion in swine was suppressed by infusion of octreotide acetate (Sandostatin®, Sandoz Pharmaceuticals, East Hanover, N.J.), and hyperglycemia was induced by concomitant infusion of 10% glucose. After a two hour induction and basel...
example 2
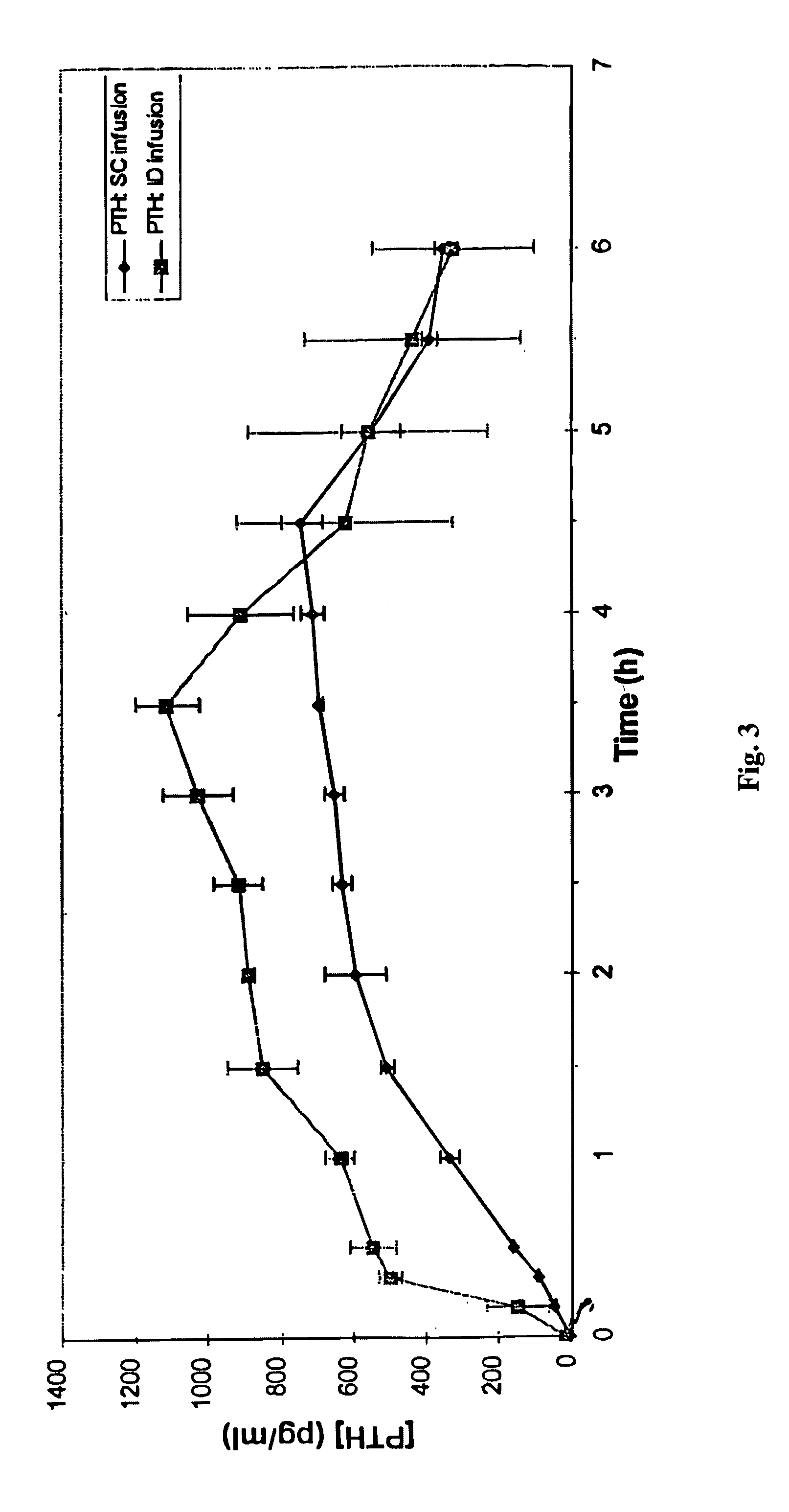
[0019] ID insulin delivery was demonstrated in swine using a hollow silicon microneedle connected to a standard catheter. The catheter was attached to a MiniMed 507 insulin pump for control of fluid delivery.
[0020] A hollow, single-lumen microneedle (2 mm total length and 200×100 μm OD, corresponding to about 33 gauge) with an outlet 1.0 μm from the tip (100 μm exposed height) was fabricated using processes known in the art (U.S. Pat. No. 5,928,207) and mated to a microbore catheter commonly used for insulin infusion (Disetronic). The distal end of the microneedle was placed into the plastic catheter and cemented in place with epoxy resin to form a depth-limiting hub. The needle outlet was positioned approximately 1 mm beyond the epoxy hub, thus limiting penetration of the needle outlet into the skin to approximately 1 mm, which corresponds to the depth of the intradermal space in swine. The patency of the fluid flow path was confirmed by visual observation, and no obstructions wer...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thick | aaaaa | aaaaa |
| depth | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com