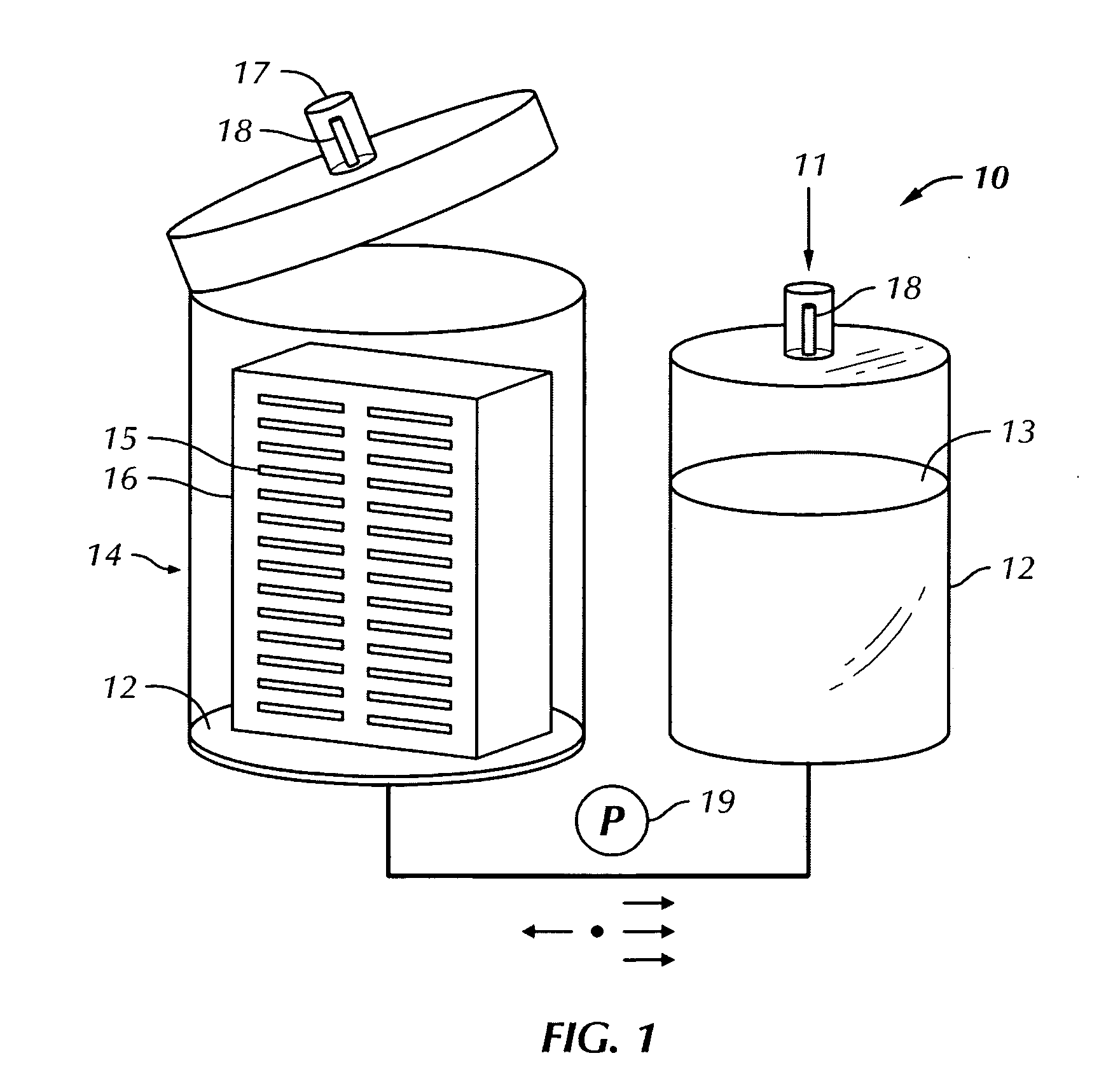
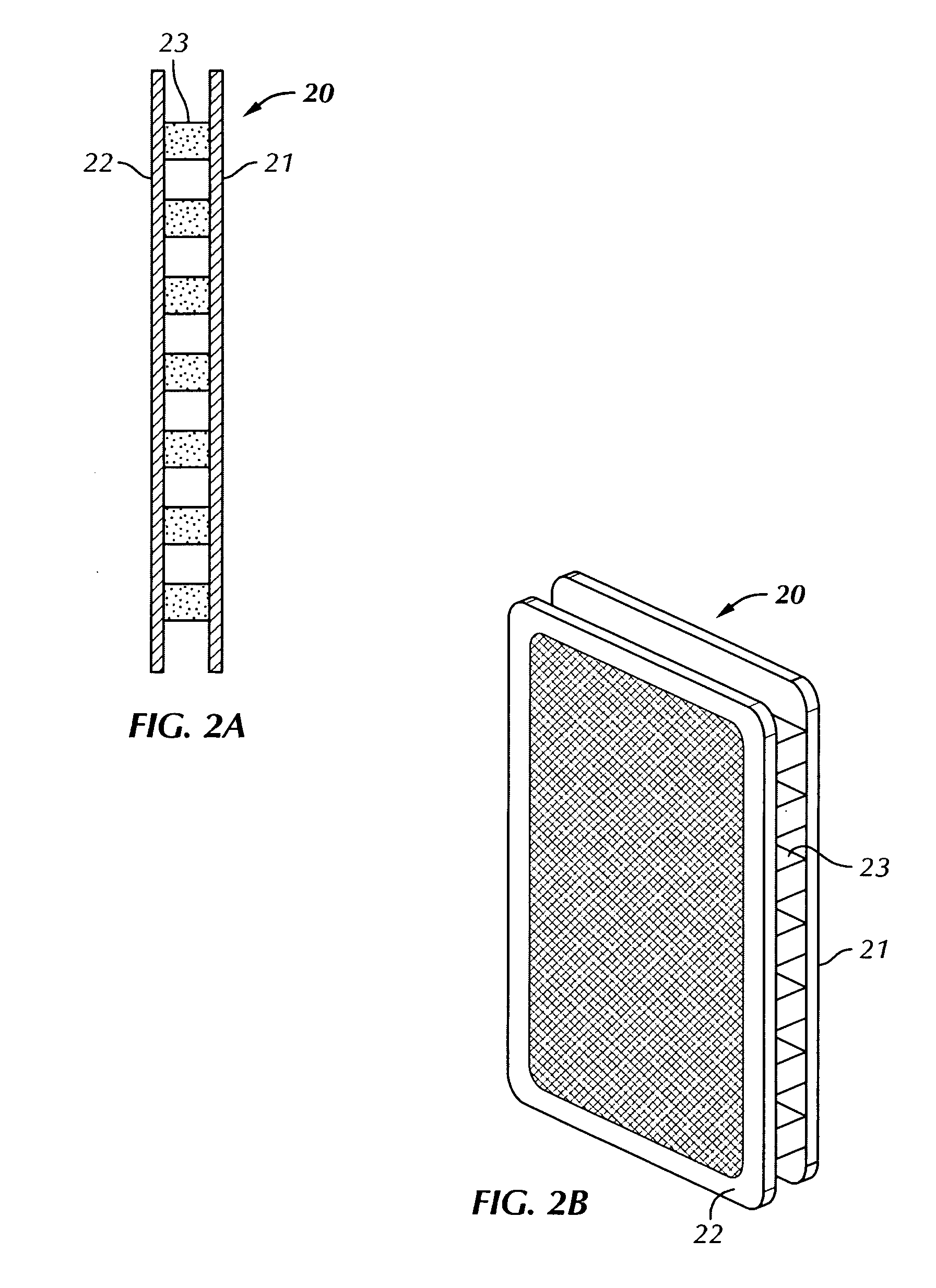
Drug testing system with bio-artificial liver
a technology of artificial liver and testing system, which is applied in the direction of biomass after-treatment, specific use bioreactor/fermenter, instruments, etc., can solve the problems of liver damage, large development cost, and inability to manage the cost in the same way
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
In Vitro Performance
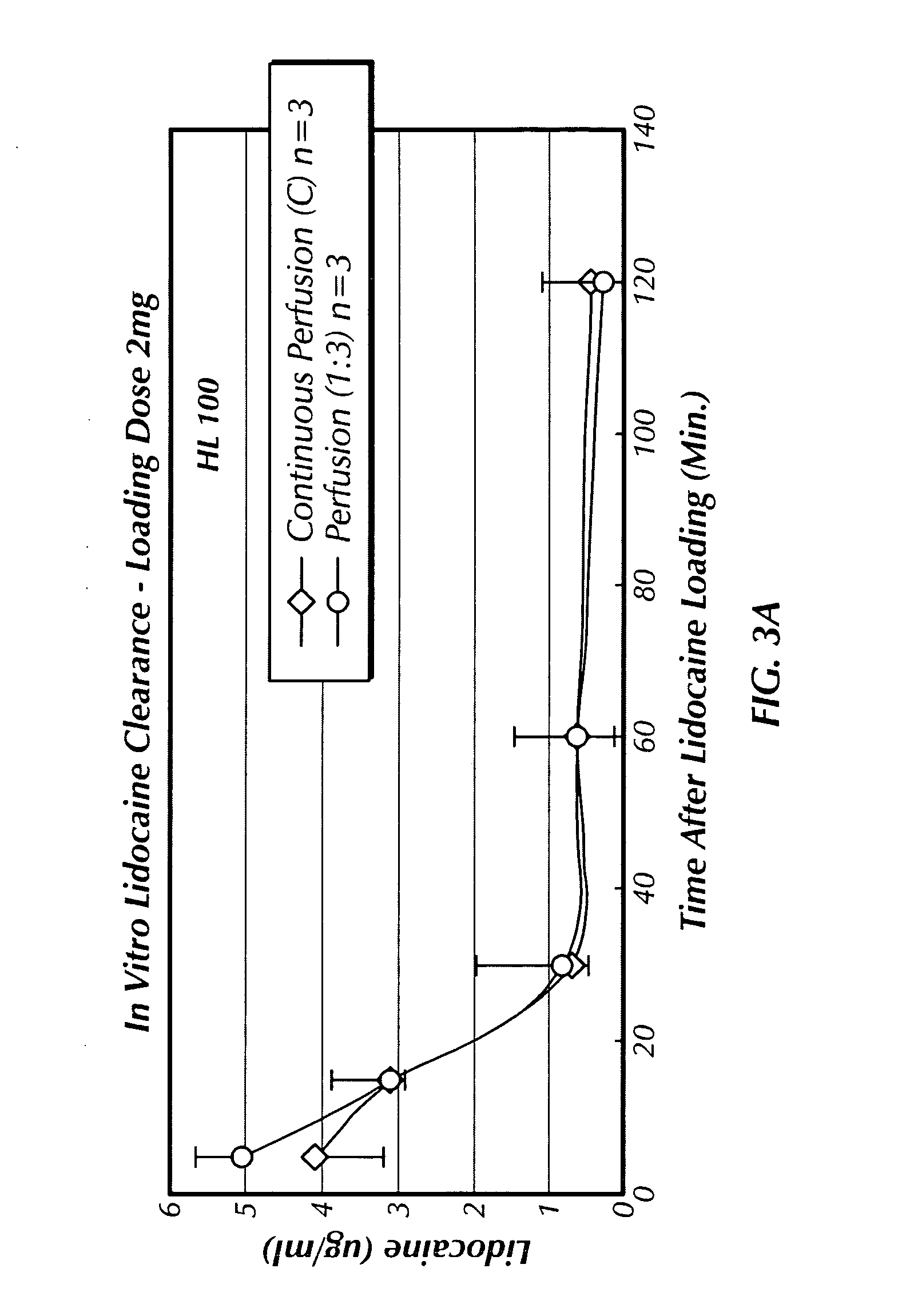
[0035] The following example illustrates the in vitro performance of the system using liver slices and forms the model for the drug testing system of the present invention. The example here shows the efficiency of liver slices to metabolize ammonia and lidocaine in the presence of drug candidate HL100.
[0036] The liver converts ammonia to urea, which is excreted into the urine by the kidneys. In the presence of severe liver disease, ammonia accumulates in the blood because of both decreased blood clearance and decreased ability to form urea. Elevated ammonia levels can be toxic, especially to the brain, and play a role in the development of hepatic encephalopathy. Accordingly, measuring ammonia clearance can assess liver function. More specifically, measuring ammonia clearance provides an indication of the operability of the present invention to metabolize compounds that may or may not be harmful to the liver.
[0037] In addition, lidocaine is a drug that can be ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com