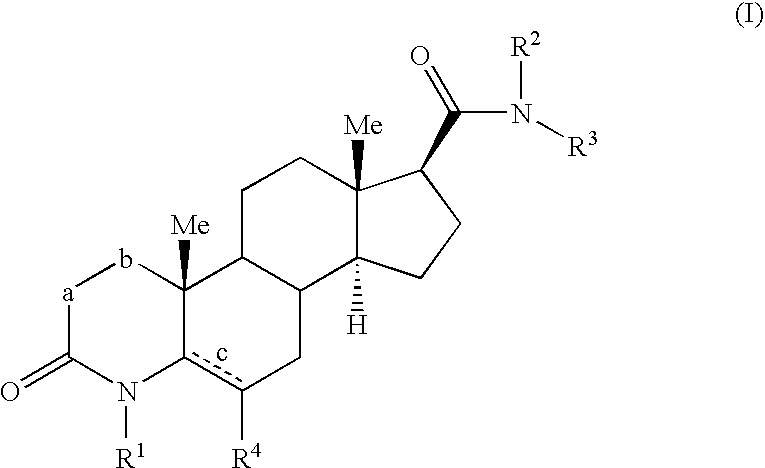
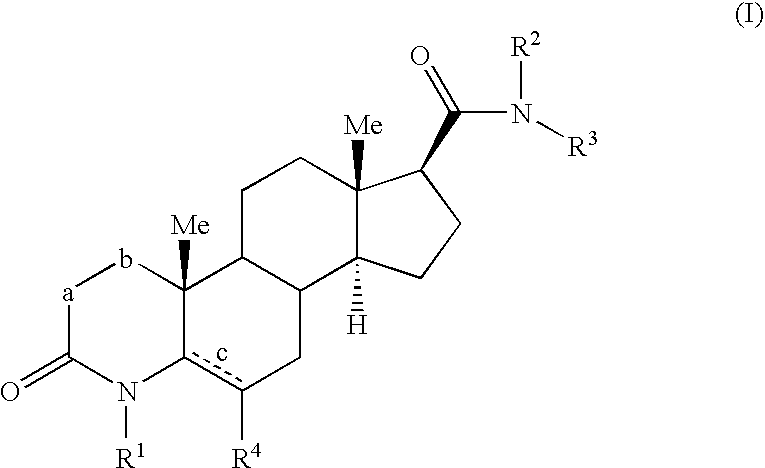
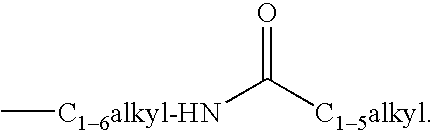
4-azasteroid derivatives as androgen receptor modulators
a technology of androgen receptor and derivatives, applied in the field of 4azasteroid derivatives, can solve the problems of limited use of androgens, increased risk of cardiovascular disease, and significant bone loss in men, and achieves the effects of reducing the risk of cardiovascular diseas
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0775]
Step A: 3-Oxo-4-aza-5α-androst-5-en-17(3-carboxylic acid methyl ester (1-2)
[0776] A mixture of 1-1, (J. Med. Chem., 29: 2298-2315 (1986)), (50.0 g, 157.5 mmol), EDC.HCl (33.2 g, 173.3 inmol) and DMAP (1.9 g, 15.8 mmol) in MeOH (300 mL) was stirred for 24 hr. The mixture was concentrated and diluted with water (1000 mL). After filtration, the solid was collected and dried to furnish the desired product 1-2 as a solid which was used in Step B without further purification.
[0777] MS calculated M+H: 332.2, found 332.2
Step B: 4-Methyl-3-oxo-4-aza-5α-androst-5-en-17β-carboxylic acid methyl ester (1-3)
[0778] To a suspension of 1-2 (7.0 g, 21.1 mmol) in 100 mL dry THF was added NaH (1.3 g, 32 nmnol) gradually. The reaction mixture was stirred at rt for 1 h. Me2SO4 (10 mL) was added in one portion. The mixture was stirred overnight. MeOH (30 mL) was gradually added. After stirring for 3 h, water (500 mL) was added. A solid precipitated out immediately. After filtration, the collec...
example 2
[0786]
S-Pyridin-2-yl)-4-methyl-6-methyl-3-oxo-4-aza-5α-androst-5-en-17β-carbothioate (2-1)
[0787] A mixture of 1-6 (1.9 g, 5.3 mmol), Ph3P (2.8 g, 10.6 mmol) and 2,2′-dipyridyl disulfide (2.3 g, 10.6 mmol) in toluene (30 mL) was stirred overnight. After solvent removal, the residue was purified by flash silica gel chromatography (10% EtOAc / 90% Hexanes to 100% EtOAc) to provide the desired product 2-1 as a solid.
[0788] MS calculated M+H: 439.2, found 439.3
N-Phenyl-4-methyl-6-methyl-3-oxo-4-aza-5α-androst-5-en-17β-carboxamide (2-2)
[0789] To a solution of 2-1 (0.05 g, 0.1 mmol) in CH2Cl2 (1 mL) was added aniline (0.03 g, 0.3 mmol) and AgOTf (0.3 g, 0.1 mmol). The mixture was stirred overnight. After filtration, the filtrate was concentrated and subsequently purified via reverse-phase chromatography [30% CH3CN / 70% H2O (with 0.02% HCl) to 95% CH3CN / 5% H2O (with 0.02% HCl) over 10 min] to afford the desired product 2-2.
[0790] MS calculated M+H: 421.3, found 421.3
[0791] Examples 2-8...
example 85
[0792]
N-(1H-benzimidazol-2-ylmethyl)-4-methyl-6-methyl-3-oxo-4-aza-5α-androst-5-en-17β-carboxamide (3-1)
[0793] A mixture of 1-6 (0.15 g, 0.4 mmol), RNH2 (0.26 g, 1.3 mmol), EDC (0.17 g, 0.87 mmol), HOAt (0.089 g, 0.65 mmol) and EtNiPr2 (0.28 g, 2.2 mmol) was stirred at rt overnight. It was concentrated and purified via reverse phase HPLC to afford the desired product 3-1 as a solid. MS calculated M+H: 475.652, found 475.3054
[0794] Examples 86 -130 in Table 2 were prepared in a similar manner as Compound 3-1 (Example 85 in Table 2), but using the appropriate amine to generate the final desired carboxamide.
TABLE 2MASSSPECTRUMMeasuredEXNR2R3NAME[M + 1]85N-(benzimidazol-2- ylmethyl)-4-methyl-6- methyl-3-oxo-4-aza-5α- androst-5-en-17β- carboxamide475.305486N-(2-fluoro-6- chlorophenylmethyl)-4- methyl-6-methyl-3-oxo-4- aza-5α-androst-5-en-17β- carboxamide487.087N-((R)- phenylmethylmethyl)-4- methyl-6-methyl-3-oxo-4- aza-5α-androst-5-en-17β- carboxamide449.188N-((S)- phenylmethylmethy...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molar density | aaaaa | aaaaa |
| Molar density | aaaaa | aaaaa |
| Molar density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com