Immunophilin ligand treatment of antiretroviral toxic neuropathy
a technology of toxic neuropathy and immunoglobulin, which is applied in the field of immunoglobulin ligand treatment of toxic neuropathy, can solve the problems of not only affecting the quality of life of patients, severely restricting viral suppression strategies, and discontinuing offending drugs in a particular patient. it prevents the development of neurotoxicity, severely restricts viral suppression strategies, and affects the quality of li
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
NRTIs Cause Dose-Dependent Neurotoxicity on Primary DRG Sensory Neuronal Cultures
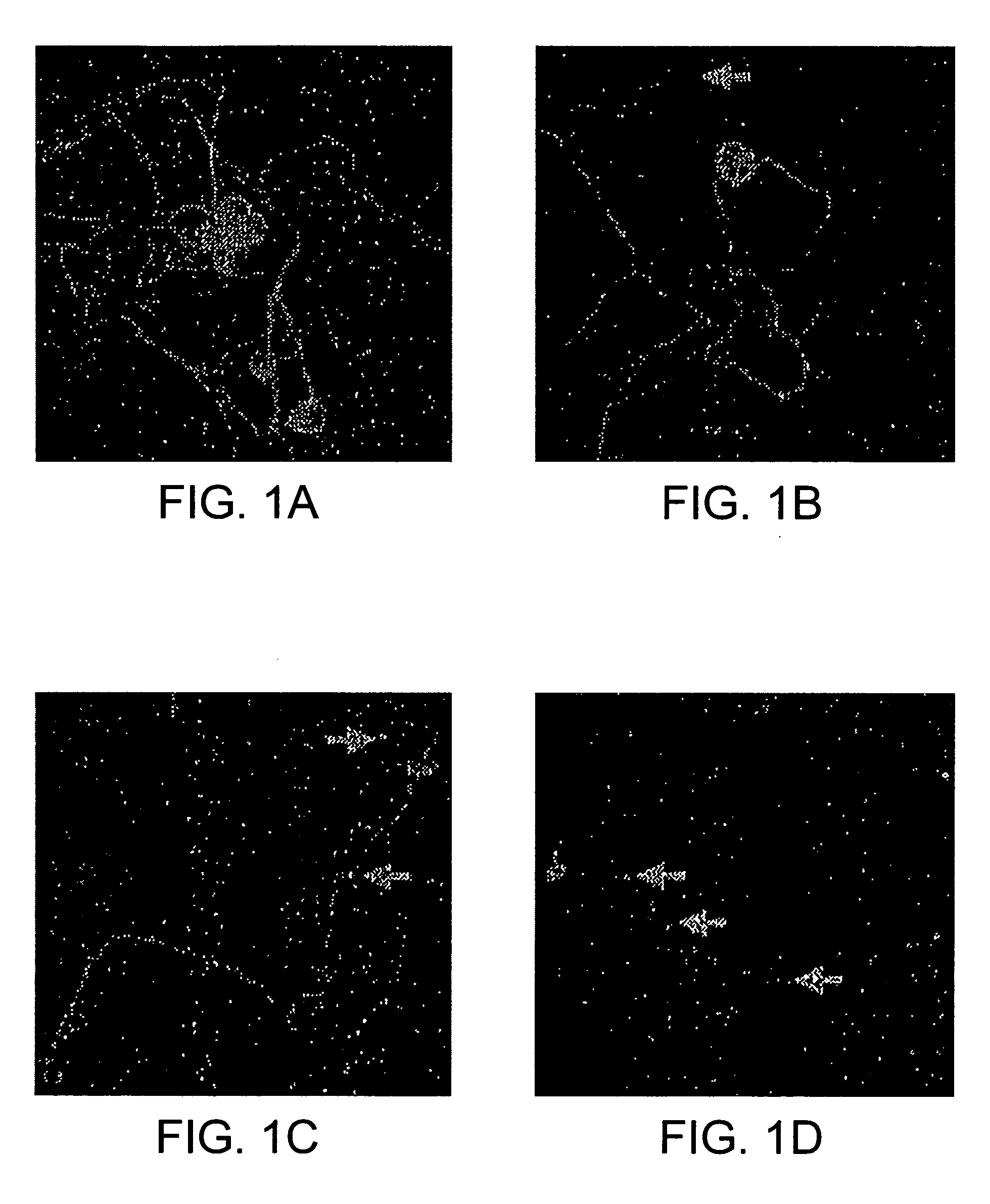
[0089] In order to assess the effects of NRTIs on established neurites primary DRG neurons were plated on a Schwann cell monolayer and allowed to extend their neurites for about 3 hours in the presence of GDNF-containing medium. At this early stage, most neurons had neurites that were at least 100 microns in length. Then added varying doses of NRTIs or vehicle control were added, before fixing and immunostaining the cultures 15 hours later. FIG. 1 (A-D) shows representative confocal microscope fields from a vehicle control culture and cultures treated with varying doses of ddC. In the control cultures, most neurons bore several neurites that were at least 200-300 microns in length and had many branching points. Morphological dose-dependent changes were noted in the ddC-treated neurons. At low ddC concentrations, varicosities were seen in the most distal portions of the neurites, away from the cell body...
example 2
FK506 Prevents the Development of ddC-Induced Neurotoxicity in Primary DRG Neurons
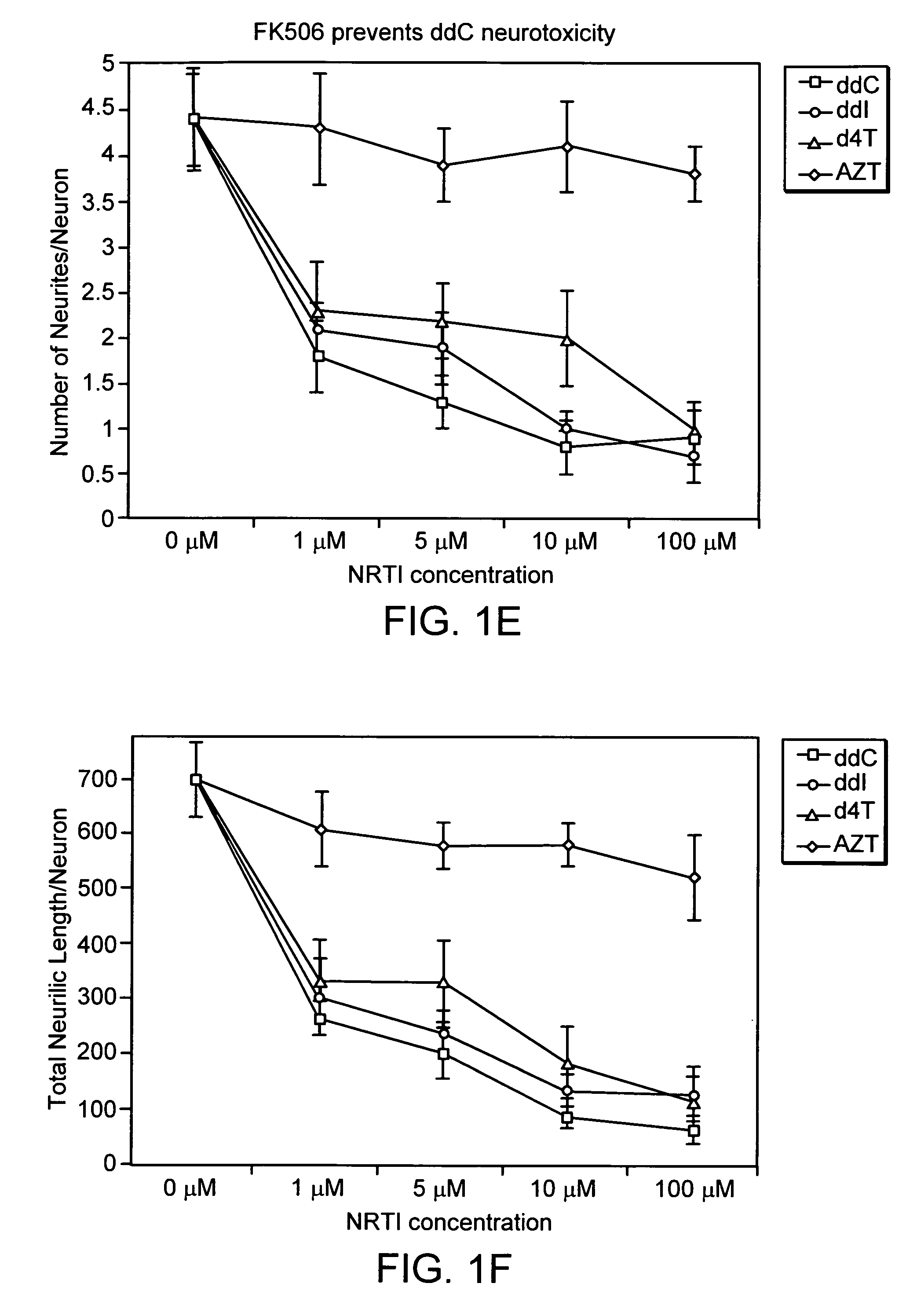
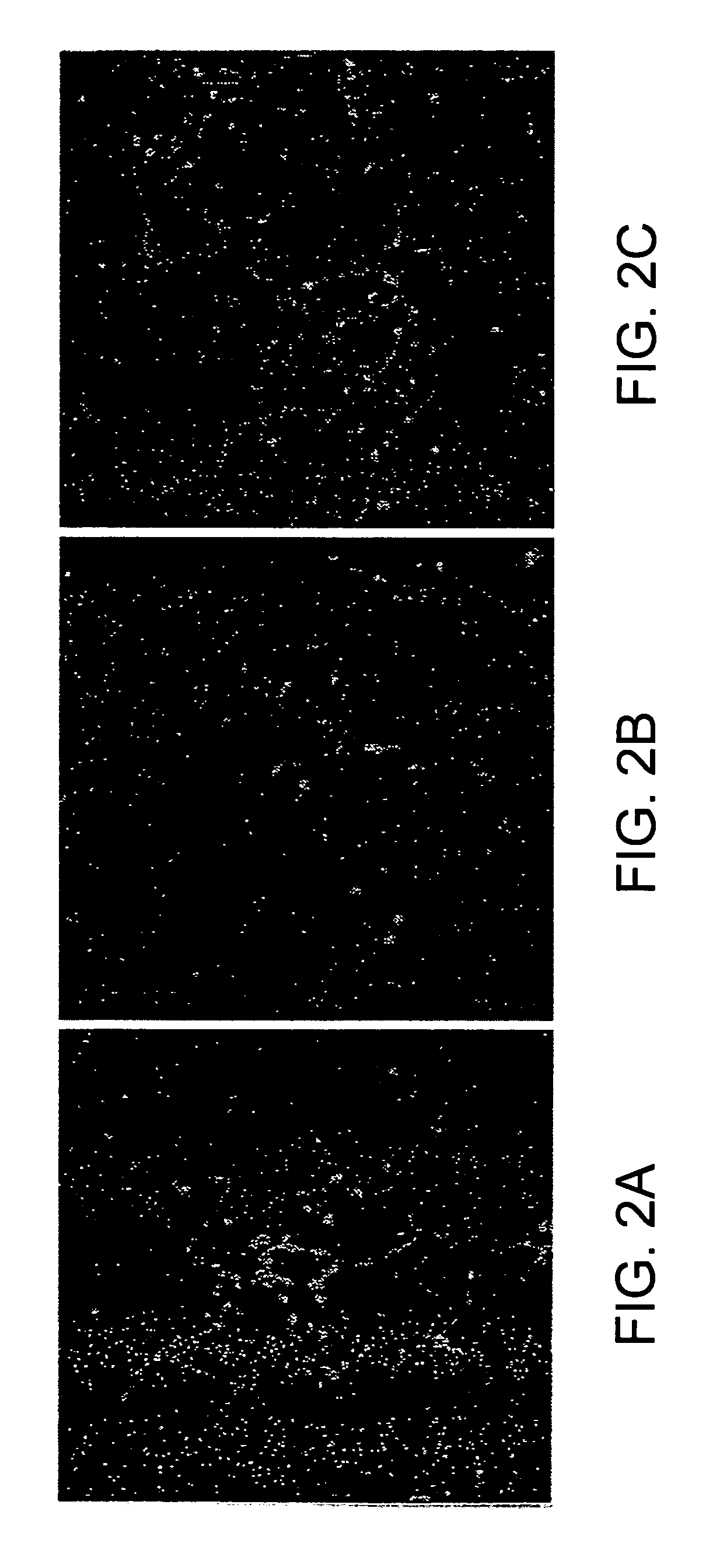
[0090] To test whether FK506 may prevent NRTI neurotoxicity in primary DRG sensory neurons, ss before, neurons were plated for 3 hours for the establishment of neurites. Then added ddC (10 μM) was added with or without FK506 and morphological changes were analyzed 15 hours later. As seen in FIG. 2 very low doses of FK506 prevented the reduction in number of neurites per neuron and total neuritic length by ddC. In contrast to FK506, CSA was not effective in preventing this neurotoxicity. In the absence of ddC, FK506 did not have any appreciable effect on the morphological parameters used in the study.
example 3
NRTI Neurotoxicity is Associated with Loss of Δ-σ in Neuronal Mitochondria Four Hours after Exposure
[0091] JC-1 is a lipophilic, cationic dye that emits green fluorescence at low concentrations when it is in monomeric form but emits a red fluorescence when aggregated. In healthy mitochondria with an intact membrane potential differential (Δ-σ), the fluorescence emission pattern of JC-1 exhibits a high red: green luminosity ratio, while in depolarized mitochondria, this ratio is low. Tetanus Toxin C-fragment was used as a live neuronal marker in our mixed DRG neuronal / Schwann cell cultures, and FCCP, a protonophore and uncoupler of oxidative phosphorylation, served as a positive control since it is a prototypic depolarizer of mitochondrial membrane. In vehicle control cultures, TTC-labeled neurons emitted mainly red and little green fluorescence (FIG. 3). The reverse was true for both the ddC- and FCCP-treated cultures, suggesting loss of mitochondrial membrane potential. FIG. 4 gra...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentrations | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com