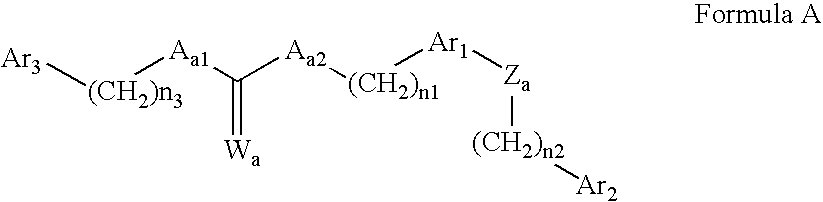
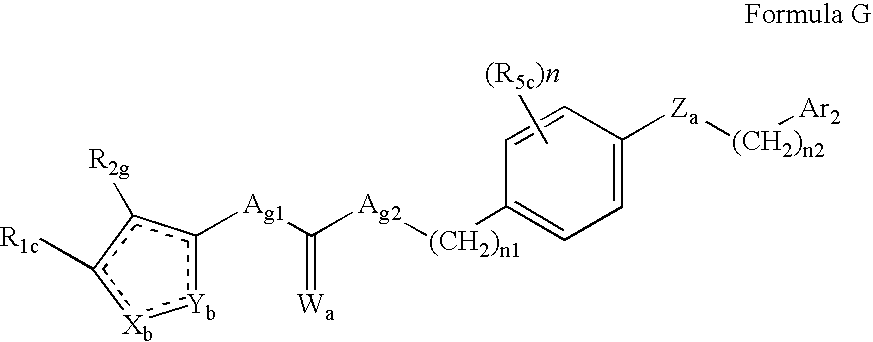
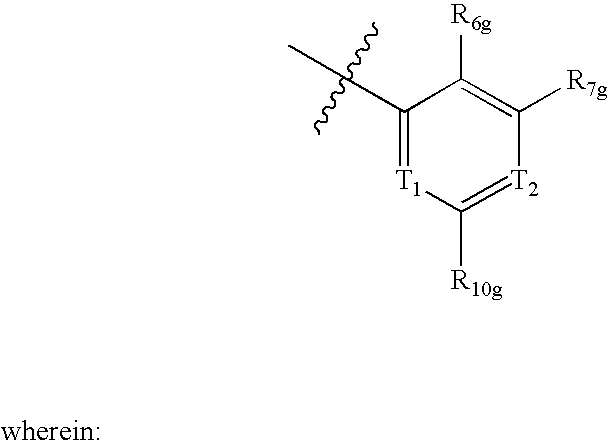
Amide derivatives as ABL modulators
a technology of amide derivatives and abl, which is applied in the direction of biocide, cardiovascular disorders, drug compositions, etc., can solve the problems of poor patient prognosis, and achieve the effects of reducing the toxicity of such inhibitory compounds, accurate targeting, and inhibiting kinase activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example a
Synthesis of Isoxazole-Amides
[0196] Compounds A1 through A240 are synthesized by methods known in the art or described herein. The structures are shown below in Table A:
TABLE ANO.STRUCTUREA1A2A3A4A5A6A7A8A9A10A11A12A13A14A15A16A17A18A19A20A21A22A23A24A25A26A27A28A29A30A31A32A33A34A35A36A37A38A39A40A41A42A43A44A45A46A47A48A49A50A51A52A53A54A55A56A57A58A59A60A61A62A63A64A65A66A67A68A69A70A71A72A73A74A75A76A77A78A79A80A81A82A83A84A85A86A87A89A90A91A92A93A94A95A96A97A98A99A100A101A102A103A104A105A106A107A108A109A110A111A112A113A114A115A116A117A118A119A120A121A122A123A124A125A126A127A128A129A130A131A132A133A134A135A136A137A138A139A140A141A142A143A144A145A146A147A148A149A150A151A152A153A154A155A156A157A158A159A160A161A162A163A164A165A166A167A168A169A170A171A172A173A174A175A176A177A178A179A180A181A182A183A184A185A186A187A188A189A190A191A192A193A194A195A196A197A198A199A200A201A202A203A204A205A206A207A208A209A210A211A212A213A214A215A216A217A218A219A220A221A222A223A224A225A226A227A228A229A...
example b
Exemplary Synthesis of Isoxazole-Amides
[0197]
[0198] In a 40 mL vial, 1 mL of thionyl chloride was added to 0.2 mmol para-substituted phenylacetic acid. The vial was capped and stirred at 80° C. for approximately three hours. The completion of the reaction was checked by TLC. The excess thionyl chloride was removed in vacuo. The residue was dissolved in dichloromethane and added to a mixture of 3-tert-butyl-isoxazol-5-ylamine (0.2 mmol) and DIEA (0.2 mmol). The reaction was stirred overnight at 45° C. The solvent was removed under vacuum and the product was purified by HPLC.
Synthesis of Compound B1: N-(3-tert-butylisoxazol-5-yl)-2-(4-(benzyloxy)phenyl)acetamide
[0199]
[0200] (4-Benzyloxy-phenyl)-acetic acid (50 mg, 0.2 mmol, 1 eq) was stirred with 1 mL of thionyl chloride at 80° C. for approximately three hours. The completion of the reaction was checked by TLC. Excess thionyl chloride was removed in vacuo, the residue was dissolved in dichloromethane and added to a mixture of 3-ter...
example c
of thiazole-amides
Synthesis of Compound C1: 2-(4-(benzyloxy)phenyl)-N-(5-methylthiazol-2-yl)acetamide
[0202]
[0203] Compound C1 was prepared in strict analogy to compound B1 using 2-amino-5-methylthiazole as starting material instead of 3-tert-butyl-isoxazol-5-ylamine.
[0204] Compounds C2 through C6 were synthesized in a manner analogous to Compound C1 using similar starting materials and reagents. The structures are shown below in Table C:
TABLE CNO.CHEMICAL STRUCTUREC1C2C3C4C5C6
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com