Phenylenediamine derivative, production method thereof and antioxidant for rubber using it as effective constituent
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
(Production of Antioxidant 1)
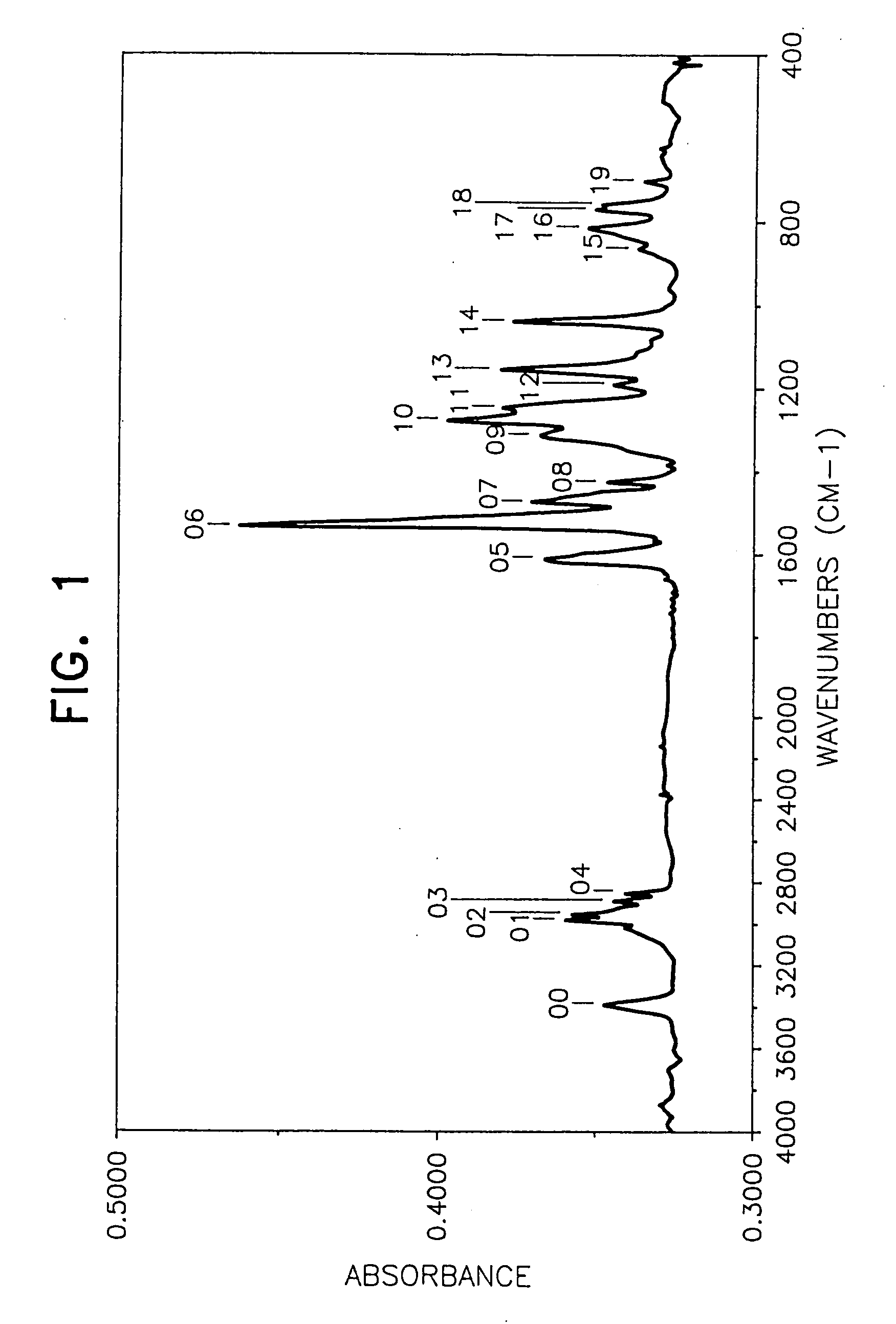
[0050] In a 500 ml three-necked flask on an oil bath were placed 52.0 g of N,N′-diphenyl-1,4-phenylenediamine (Nocrac DP, a trade name made by Ouchi Shinko Kagaku K.K.) and 74.9 g of 4-(1-propenyl)-1,2-dimethoxybenzene and after sufficiently replacing the atmosphere with a nitrogen gas with stirring, the temperature of the oil bath was increased to 140° C. Thereafter, 5 g of concentrated sulfuric acid was added dropwise from a dropping funnel to the mixture over a period of about 30 minutes, and when after finishing the addition, the reaction was further continued for about 15 hours, the viscosity of the reaction mixture was increased with the passage of time. After the reaction was over, toluene was added to the reaction mixture to form a toluene solution, then the toluene solution was poured into an excessive amount of an aqueous sodium hydroxide solution, followed by stirring, and then a lower layer (aqueous solution layer) formed was separated by a...
example 2
(Production of Antioxidant 2)
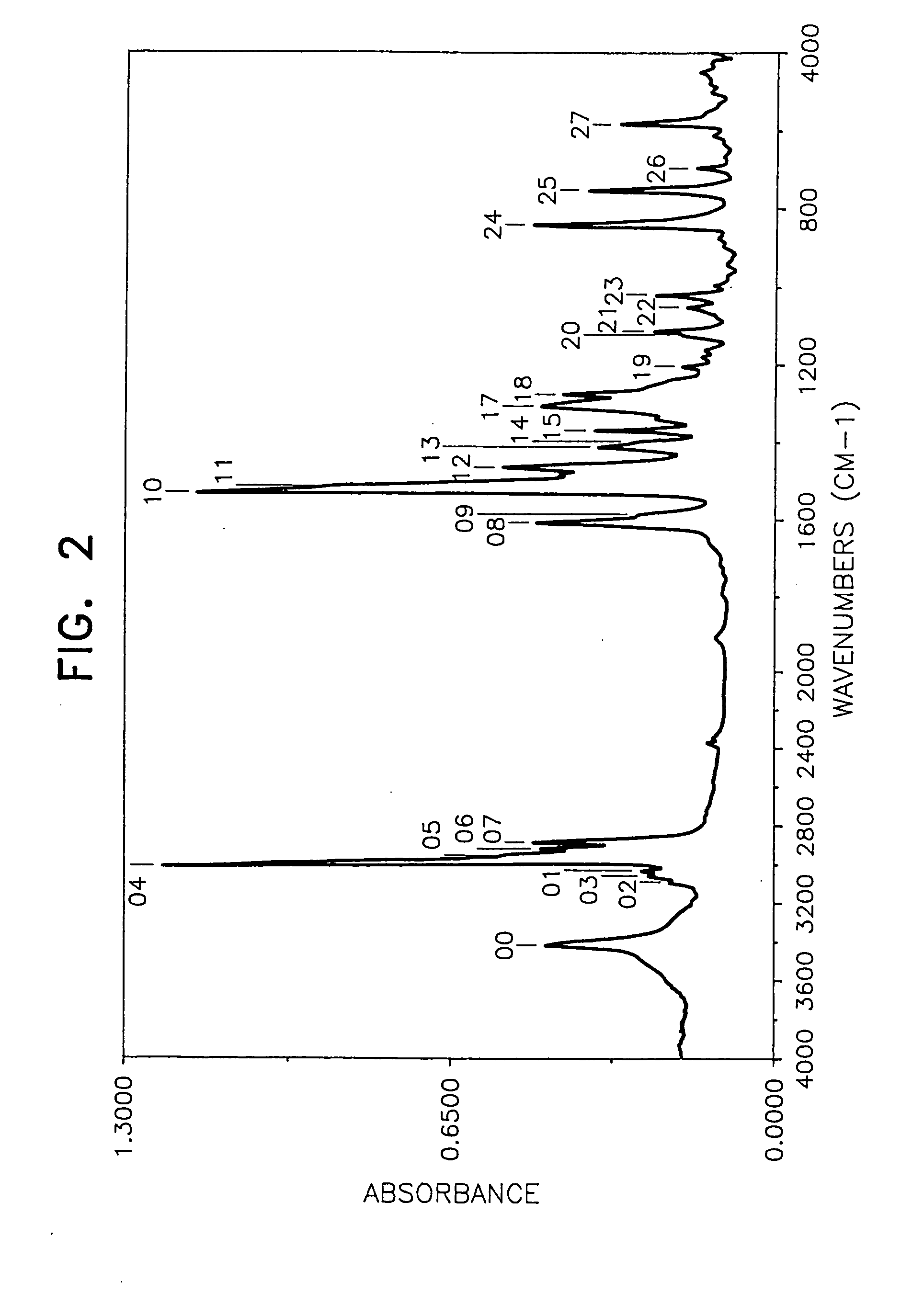
[0055] By following the same procedure as in Example 1 except that the amount of N,N′-diphenyl-1,4-phenylenediamine was changed to 78.0 g and 99.5 g of 4-tert-butylstyrene was used in place of the 4-(1-propenyl)-1,2-dimethoxybenzene, 139.0 g (yield 80%) of the desired product was obtained.
[0056] The weight loss of the product at 175° C. for 40 hours was lower than 10% (6.2%) and the behaviors of the product by the TLC method were substantially the same as those in Example 1. Mn (by a GPC method using tetrahydrofuran as the solvent): 700 FI-IR (measured by casting on a KRS-5 crystal plate): FIG. 2 1H-NMR (CDCl3, 25° C.): [0057] near 1.22 ppm (CH3 group) [0058] near 7.1 ppm (aromatic H)
Presumed Structure:
example 3
(Production of Antioxidant 3)
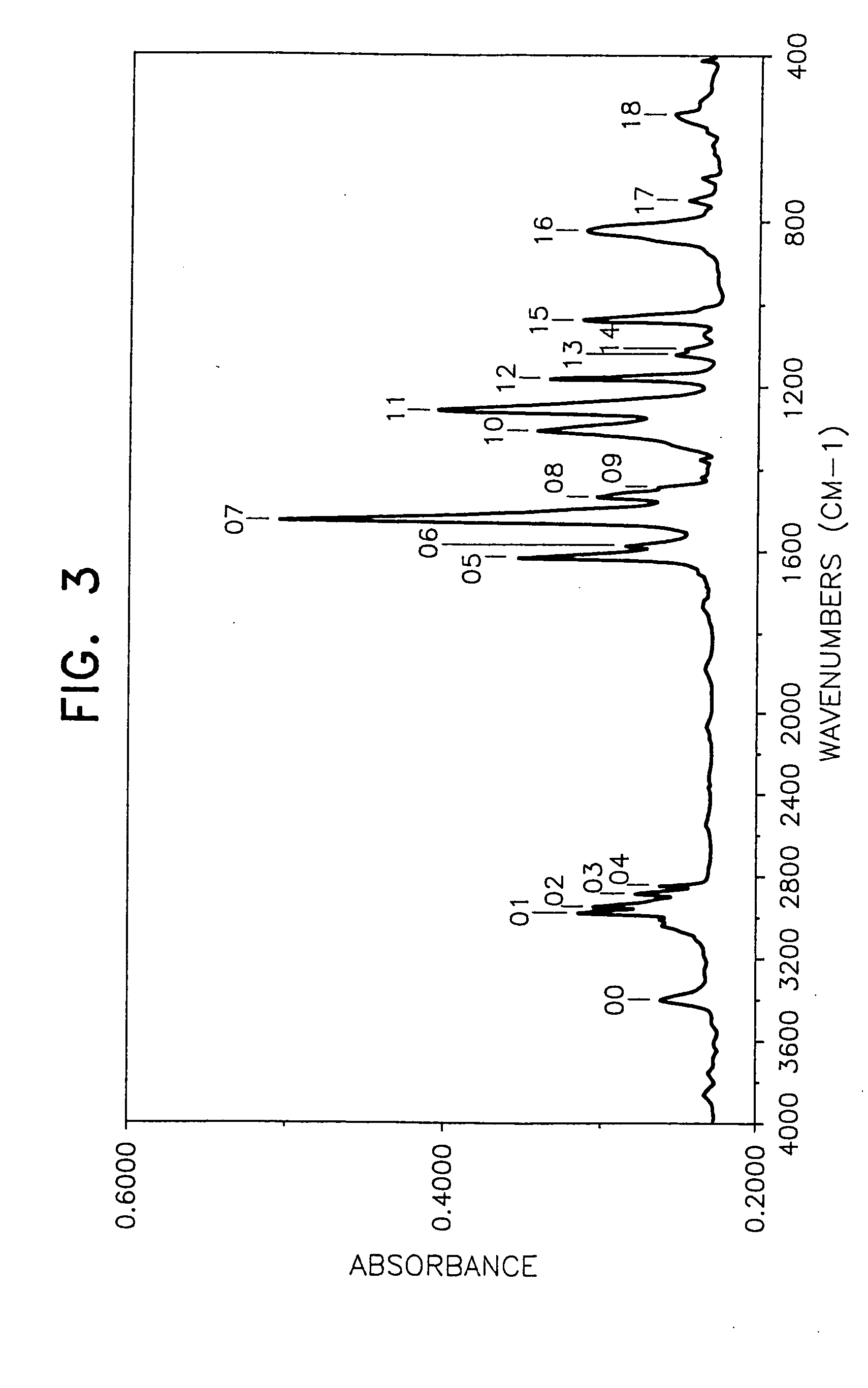
[0059] By following the same procedure as in Example 1 except that 62.2 g of anethol-(p-methoxypropenylbenzene) was used in place of the 4-(1-propenyl)-1,2-dimethoxybenzene, 94.6 g (yield 85%) of a blackish brown desired product was obtained.
[0060] The weight loss of the product at 175° C. for 40 hours was lower than 10% (8.6%) and the behaviors of the product by the TLC method were substantially the same as those in Example 1. Mn (by a GPC method using tetrahydrofuran as the solvent): 704 FI-IR (measured by casting on a KRS-5 crystal plate): FIG. 3 1H-NMR (CDCl3, 25° C.): [0061] near 0.88 ppm (CH3 group) [0062] near 3.8 ppm (OCH3 group) [0063] near 6.8 to 7.2 ppm (aromatic H)
Presumed Structure:
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com