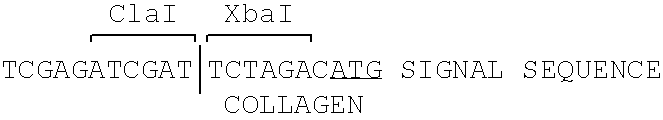Production of collagen in the milk of transgenic mammals
a technology of transgenic mammals and collagen, which is applied in the field of transgenic nonhuman mammals, can solve the problems of complex process by which collagen is expressed, processed and ultimately assembled into mature collagen fibers, and the mammalian cellular expression system is not entirely satisfactory for recombinant protein production, and it is unpredictable whether this technology could be extended to the expression of multimeric proteins requiring extensive post-translational modification and assembly, such as collagen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
Vectors for Collagen Expression
[0070] a. Proα1(l) Collagen cDNA Based Expression Vector
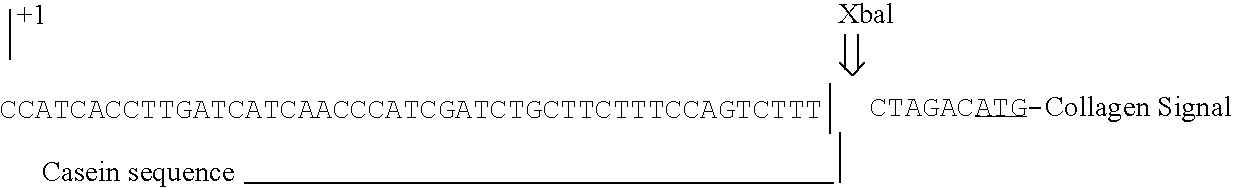
[0071] A plasmid vector was constructed containing the bovine αS1-casein 5′-flanking region including the proximal promoter operably linked to a cDNA sequence encoding the human proα(I) collagen gene, which is in turn operably linked to a 3′-flanking sequences derived from the human genomic collagen gene. The fusion product of the collagen cDNA [XbalSalI fragment] and the casein promoter at a Clal site yields the following nucleotide sequence:
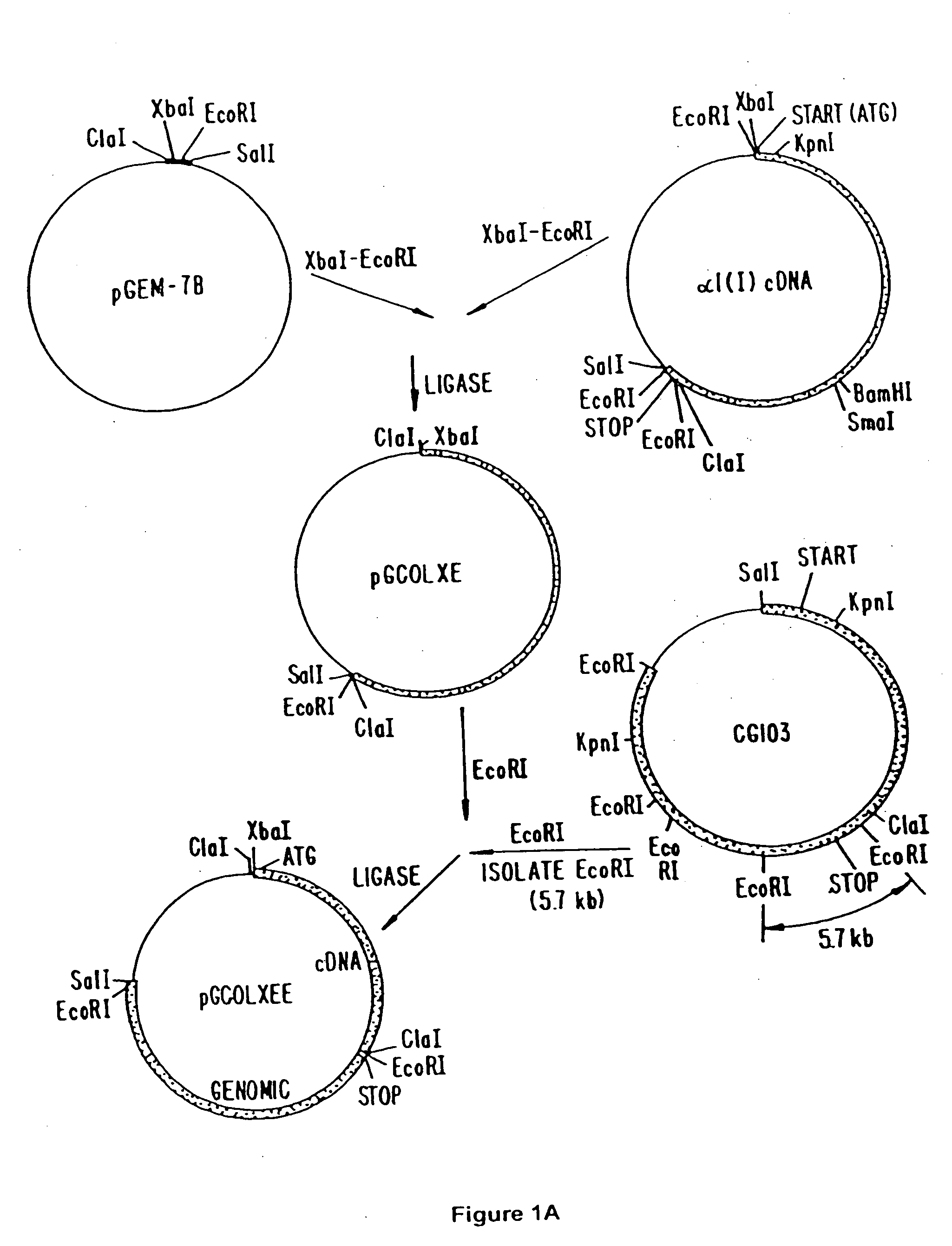
[0072] The XbaI-EcoRI fragment (4363 bp) of a collagen cDNA was subcloned into the XbaI-EcoRI site of pGEM-7B, giving rise to the pGCOLXE plasmid (FIG. 1). This collagen cDNA fragment lacks the region encoding for the last 10 amino acids of the protein. The full-length coding region of the human proα1(I) collagen was reconstituted by fusing a 5.7 kb EcoRI fragment (Schnieke et al., Proc. Natl. Acad. Sci. USA 84, 764-768 (1987)) derived from the GC103 geno...
example 2
Expression of Constructs in Mammary Cell Culture
[0087] This example shows the feasibility of expressing, assembling and secreting an α1(I) procollagen in mammary gland cells, a cell type that does not normally express this gene. The cDNA and genomic vectors described above were transfected in their circular form into the mouse mammary epithelial cell line HC11 (Ball et al., EMBO 7, 2089-2095 (1988)). The cells were maintained as monolayers in RPMI 1640 (10% FCS, 2 mM L-Glutamine, 50 μg / ml gentamicin, 5 μg / ml insulin, 10 ng / ml EGF). 30-40 μg of each construct together and a hygromycin-resistance cotransfecting plasmid were complexed with 50 μg lipofectine (Gibco) and allowed to fuse with 2-3×106 cells. After 48 hr of growth in normal medium, selection medium was applied to select for stable transfectants. Two independent transfection rounds have been performed, and resistant colonies were scored after about 2 weeks (Table 1).
TABLE 1TRANSFECTION OF COLLAGEN EXPRESSION VECTORSNUMBER...
example 3
Production of Transgenic Animals Expressing α1(I) Procollagen
[0098] (1) Transgenesis
[0099] Collagen transgene fragments were excised from the three vectors described in Example 1 by NotI digestion and purified by 0.65% agarose gel electrophoresis and electroelution. (FIG. 2, panel A). Fertilized mouse eggs (CBA / BrAxC57B1 / 6) were microinjected (with 100-200 copies of the fragment) and transferred into pseudo-pregnant females as described (Hogan et al., supra). Total genomic DNA was prepared from a short segment of mouse tail to check for integration of the injected DNA. EcoRI-digested tail DNA was analyzed by Southern blotting (Sambrook et al., supra). The probe used to check for integration of the transgene was a 300 bp NcoI-NsiI fragment, spanning the region from −680 to −250 (relative to the major transcription start site) of the bovine αS1-casein gene. The probe was labeled with 32P using random hexanucleotide primers (Sambrook et al., supra). The numbers of transgenic mice con...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com