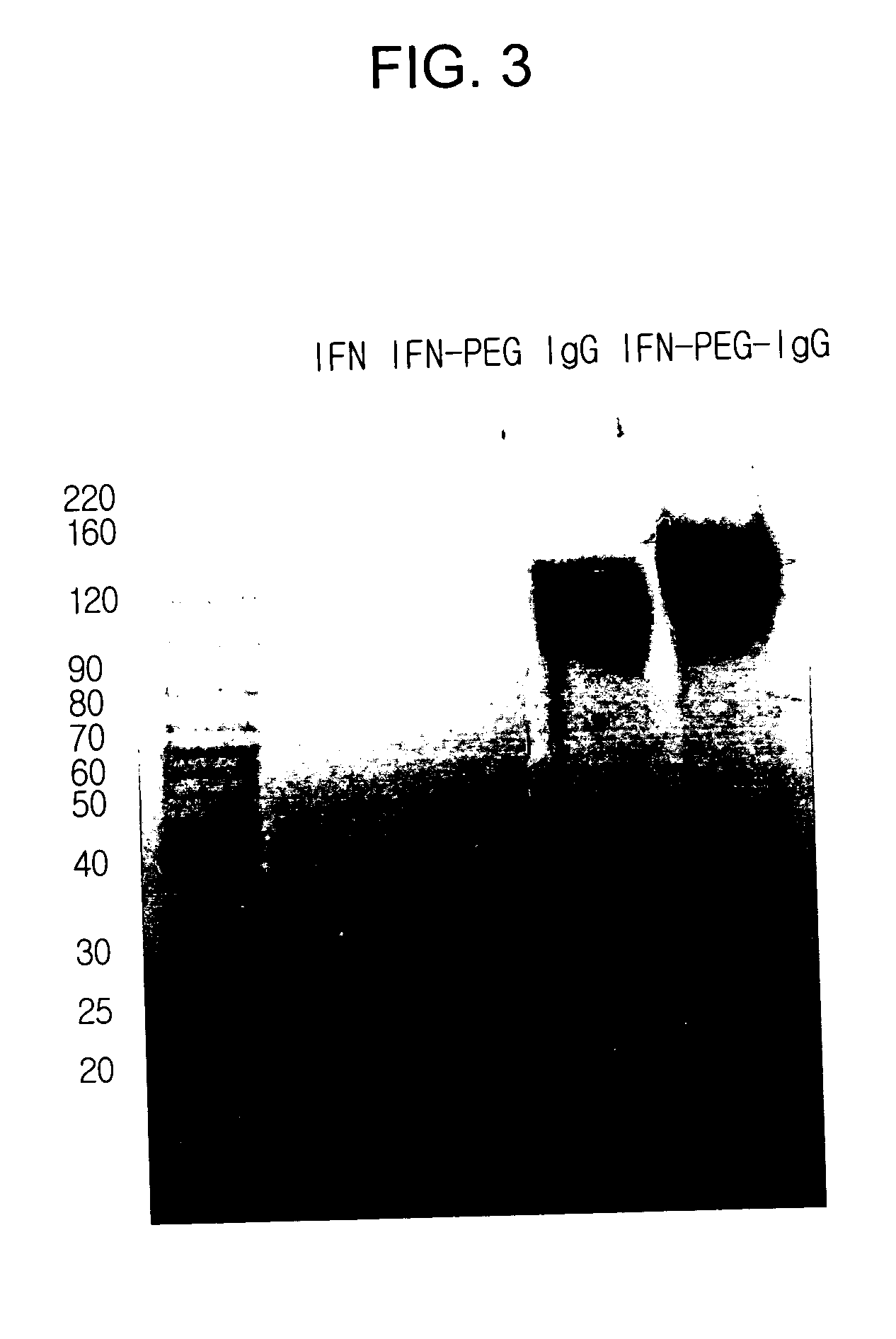Physiologically active polypeptide conjugate having prolonged in vivo half-life
a polypeptide and in vivo half-life technology, applied in the field of long-acting proteins having a prolonged in vivo half-life, can solve the problems of reducing the activity and production yield of an active substance, affecting the activity of the active substance, so as to prolong the circulating half-life of a physiologically active polypeptide, and enhance the in vivo stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
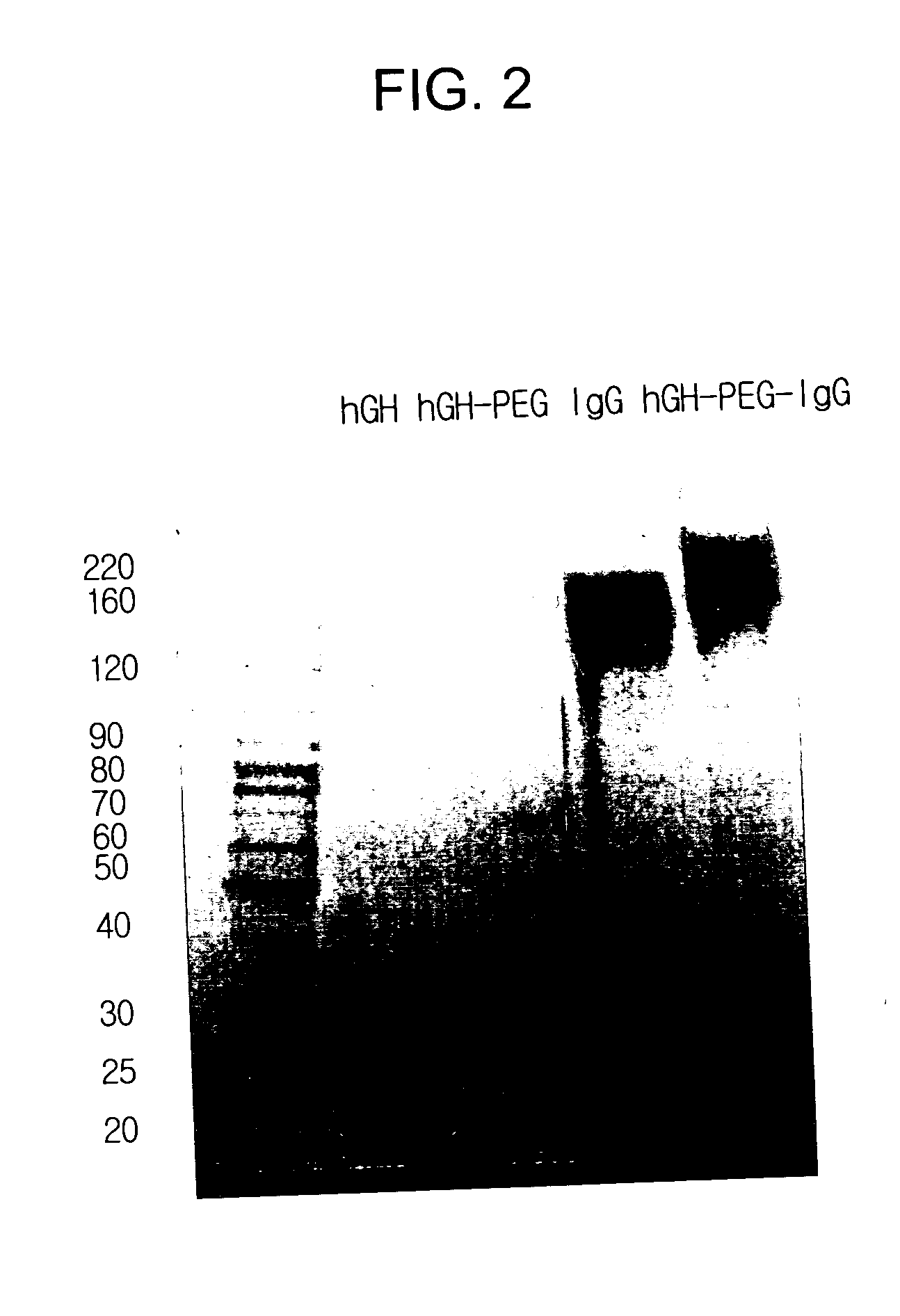
Preparation of hGH-PEG-IgG conjugate I
(Step 1) Preparation of hGH-PEG Complex
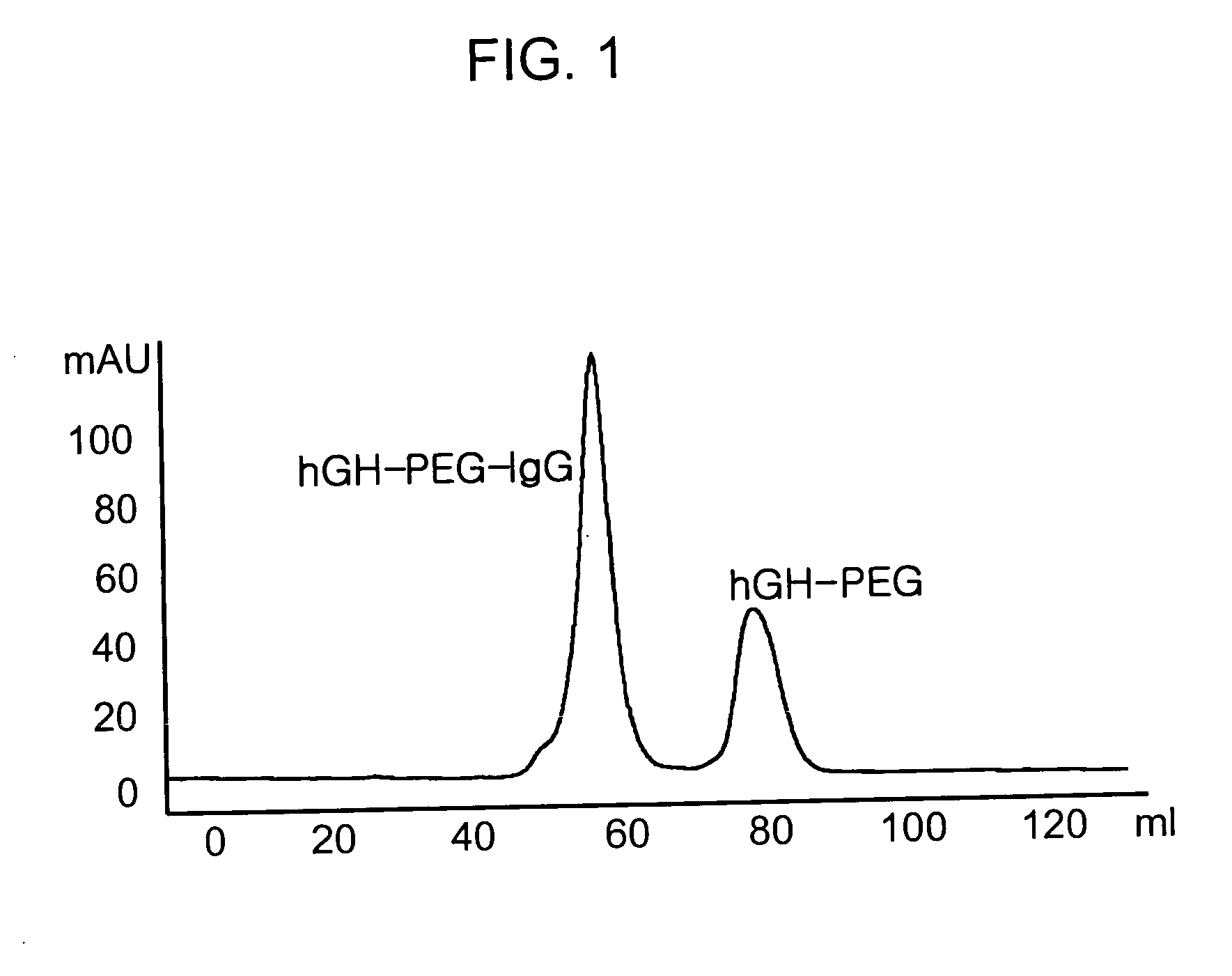
[0061] Human growth hormone (hGH, M.W. 22 kDa) was dissolved in 100 mM phosphate buffer solution to a concentration of 5 mg / ml, and polyethylene glycol containing aldehyde groups at both ends (ALD-PEG-ALD, Shearwater Inc, USA) which has a molecular weight of 3.4 kDa was added to the resulting buffer solution in an amount corresponding to an hGH:PEG molar ratio of 1:1, 1:2.5, 1:5, 1:10 or 1:20. Sodium cyanoborohydride (NaCNBH3, Sigma) was added thereto to a final concentration of 20 mM as a reducing agent, and the reaction mixture was stirred at 4° C. for 3 hours. To separate an hGH-PEG complex in which PEG is selectively linked to the terminal amino residue of hGH in a molar ratio of 1:1, the reaction mixture was subjected to Superdex® (Pharmacia, USA) size exclusion chromatography. The hGH-PEG complex was eluted and purified from the column with 10 mM potassium phosphate buffer (pH 6.0) to remove contam...
example 2
Preparation of hGH-PEG-IgG Conjugate II
(Step 1) Preparation of I2G-PEG Complex
[0064] IgG (Green Cross, Korea) was dissolved in 100 mM phosphate buffer to a concentration of 15 mg / ml, and 3.4 kDa of ALD-PEG-ALD (Shearwater Inc, USA) was added to the resulting buffer solution in an amount corresponding to an IgG:PEG molar ratio of 1:1, 1:2.5, 1:5, 1:10 or 1:20. NaCNBH3 was added thereto to a final concentration of 20 mM as a reducing agent, and the reaction mixture was stirred at 4° C. for 3 hours. To separate IgG-PEG complex in which PEG is selectively linked to the terminal amino residue of IgG in a molar ratio of 1:1, the reaction mixture was subjected to Superdex® (Pharmacia, USA) size exclusion chromatography. The IgG-PEG complex was eluted and purified from the column with 10 mM potassium phosphate buffer (pH 6.0) to remove contaminants such as unmodified IgG, unreacted PEG and dimmeric by-products having two molecules of IgG linked at both ends of PEG. The purified IgG-PEG c...
example 3
Preparation of IFN α-PEG-IgG Conjugate
[0066] An IFN α-PEG-IgG conjugate was prepared and purified according to the same method described in Example 1, except that interferon alpha 2b (IFN α 2b, M. W. 20 kDa) was employed instead of hGH and the IFN α 2b:ALD-PEG-ALD (M.W. 3.4 kDa) molar ratio was 1:5.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com