Vectors for molecule delivery to CD11b expressing cells
a technology of cd11b and molecule, applied in the direction of lyase, immunological disorders, antibody medical ingredients, etc., can solve the problems of papc specifically dendritic cells, no vector has been shown to be exclusively targeted to papc particularly to dendritic cells and more particularly
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
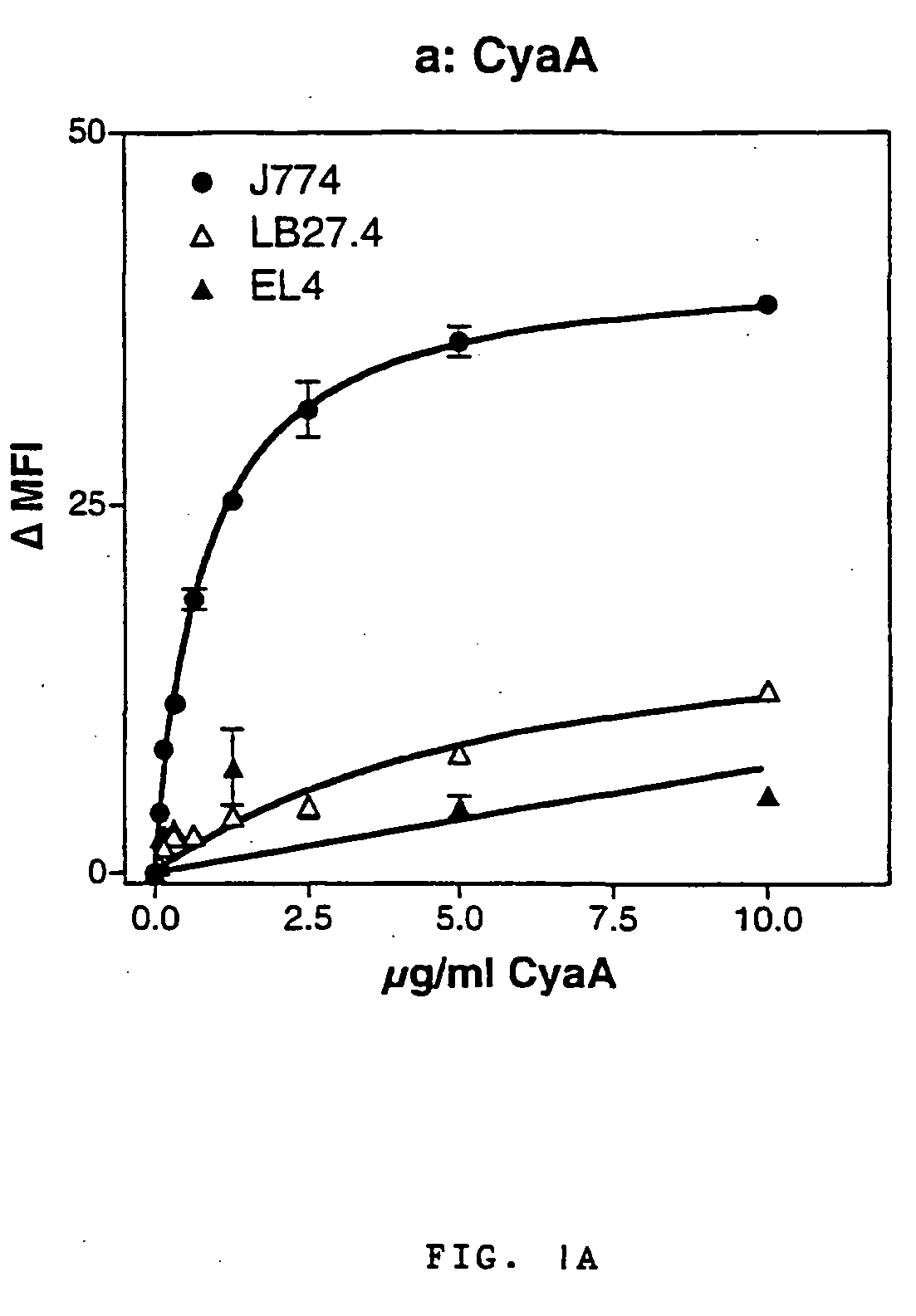
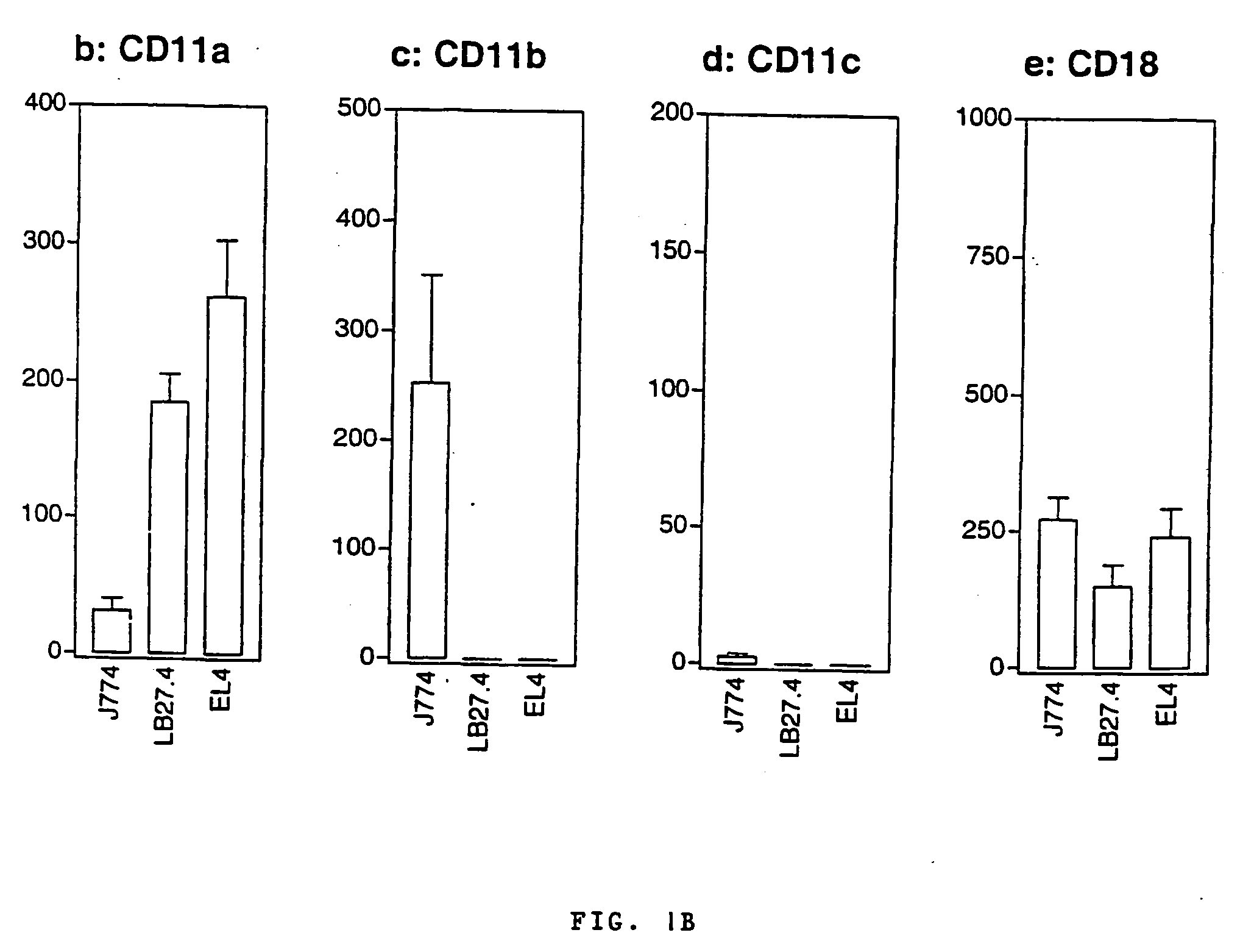
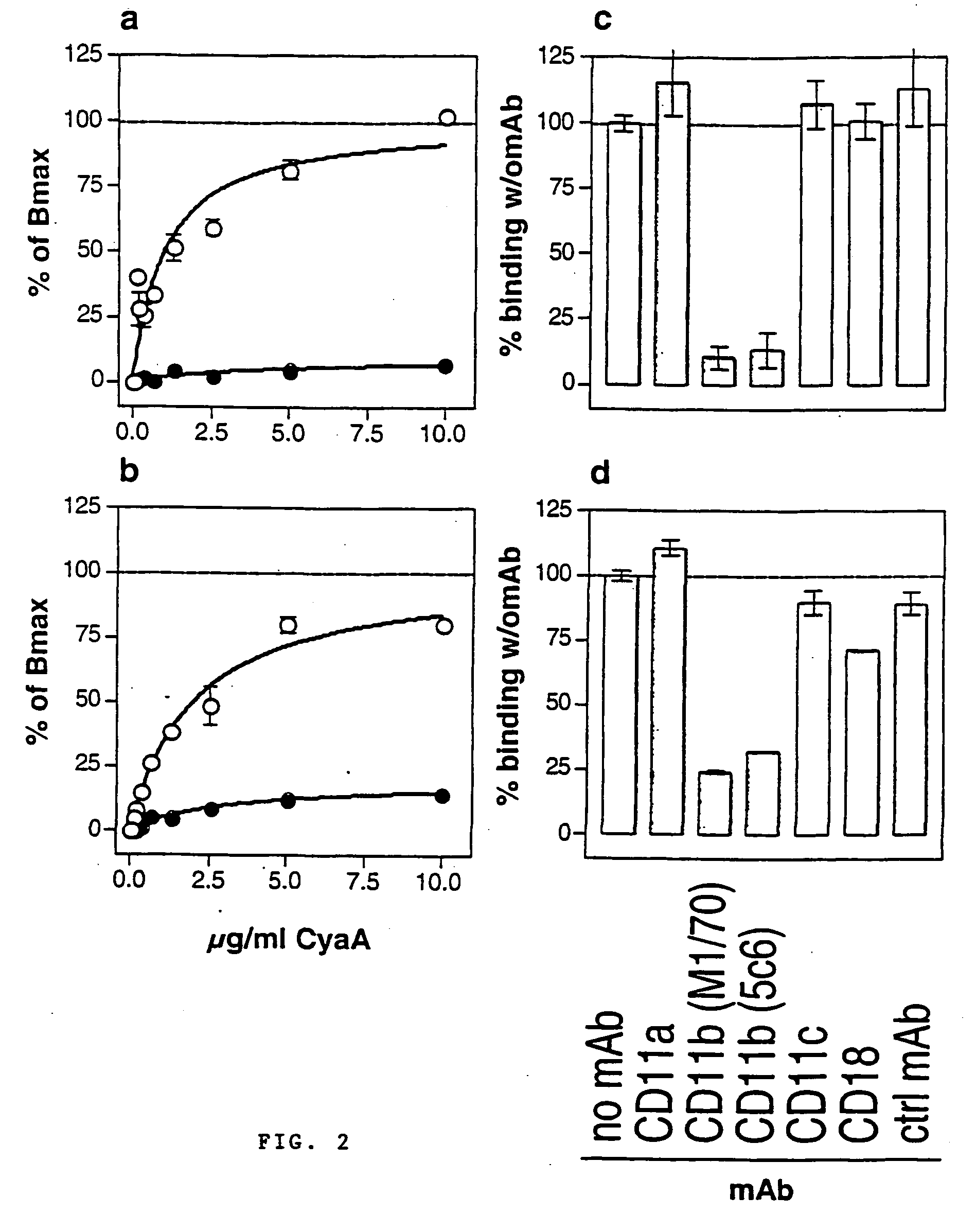
A. The Bordetella Adenylate Cyclase Toxin Interacts Specifically with the αMβ2 Integrin (CD11b / CD18)
A.1 Materials and Methods
A.1.1 Recombinant Toxins and Antibodies
[0152] Protocol for CyaA production has already been described elsewhere [Karimova, et al, 1998]. CyaA toxins were produced in E. coli BLR strain harboring an expression plasmid, pCACT3, which carries the cyaA structural gene CyaA under the lacUV5 promoter and the cyaC accessory gene required for activation of the protoxin. After solubilization in 8M urea, Hepes-Na 20 mM, pH 7.5, CyaA was purified to more than 95% homogeneity (as judged by SDS-gel analysis, not shown) by sequential DEAE-Sepharose and Phenyl-Sepharose. A recombinant detoxified CyaA toxin, CACTE5-Cys-Ova, harboring a unique cysteine inserted within the genetically inactivated catalytic domain was constructed by inserting an appropriate double strand oligonucleotide between the BsiwI and KpnI sites of pCACT-Ova-E5 [Guermonprez et al, 2000]. In the resul...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Immunogenicity | aaaaa | aaaaa |
| Toxicity | aaaaa | aaaaa |
| Catalyst | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com